The Long Road to Approval for Gene Therapies
We’ve come a long way from the first gene therapy clinical trial in 1990, but for all the hurdles overcome and advancements made, timelines remain one of the most prominent pain points for drug innovators, contract development and manufacturing organizations (CDMOs), and especially patients desperately waiting for treatment. The long road starts with high investment costs for extensive research and development, followed by a clinical trial that could take several years due to the manufacturing work itself, patient recruitment and supply chain challenges, and the regulatory approval process.
While there’s no magic wand that can expedite these activities, access to current market data can support biopharma companies and their partners in planning for the success of their gene therapy projects. The outsourcing relationships between innovators and manufacturers are critical parts of drug development, and mismatches between expectations and capabilities can cause further delays for these much needed therapies. Biopharma companies must carefully consider a CDMO’s reputation, ability to meet their project’s needs, and future goals for their project before making a final decision.
Insights from Sponsors’ Experiences with CDMOs
With the right information in hand, innovators can make informed decisions regarding CDMO selection and CDMOs can position themselves as the ideal gene therapy manufacturing partner. That’s where data from ISR’s new Gene Therapy CDMO Benchmarking research can help. We surveyed 77 respondents from biopharma companies of all sizes to understand their experience with outsourcing gene therapy manufacturing projects and how they rate the services of the providers they worked with.
The full report explores drug innovators’ outsourcing practices and philosophies, what drives their service provider selection, and how individual CDMOs performed across 27 performance attributes specific to gene therapy development and manufacturing. Biopharma companies, CDMOs, consultants, and financial sponsors can utilize these data to plan strategically for the success of their gene therapy endeavors. This article will explore our respondents’ feedback on some key timelines at play after a sponsor decides to partner with a CDMO, followed by the most important attributes sponsors seek in a gene therapy CDMO.
Gene Therapy Project and Program Activities Timelines
It can take 6 to 12 years for a gene therapy to progress from Investigative New Drug (IND) submission to approval1. To home in on a piece of that timeline, we asked respondents how long it takes to sign a Master Service Agreement (MSA) after first reaching out to a manufacturer. They revealed an average of 8 months from the sponsor’s initial contact with a CDMO to signing an MSA: one-third (33%) of respondents indicated that it takes Between 3 and 6 months, roughly one-fifth indicated a duration of Between 6 and 12 months (22%), or Between 12 and 18 months (21%).
We also asked participants how long it takes to get their program underway once they’ve chosen a CDMO outsourcing partner. They relayed that it takes an average of about 6 months to start their gene therapy program specific activities after signing an MSA: a third of respondents (32%) indicated Between 3 and 6 months, 27% stated Between 1 and 3 months, and about one-fifth (23%) waited Between 6 and 12 months. Only 10% of respondents waited longer than 1 year to start their gene therapy program activities.
“On a typical outsourced gene therapy project, how quickly were you able to activate a project (the timeframe from the initial contact to Master Service Agreement is signed) with the chosen CDMO?” (n=74, “Don’t know” responses removed)
“Continuing to think about a typical outsourced gene therapy project, how quickly were you able to start the program specific activities once the MSA is signed with the chosen CDMO?” (n=74, “Don’t know” responses removed)
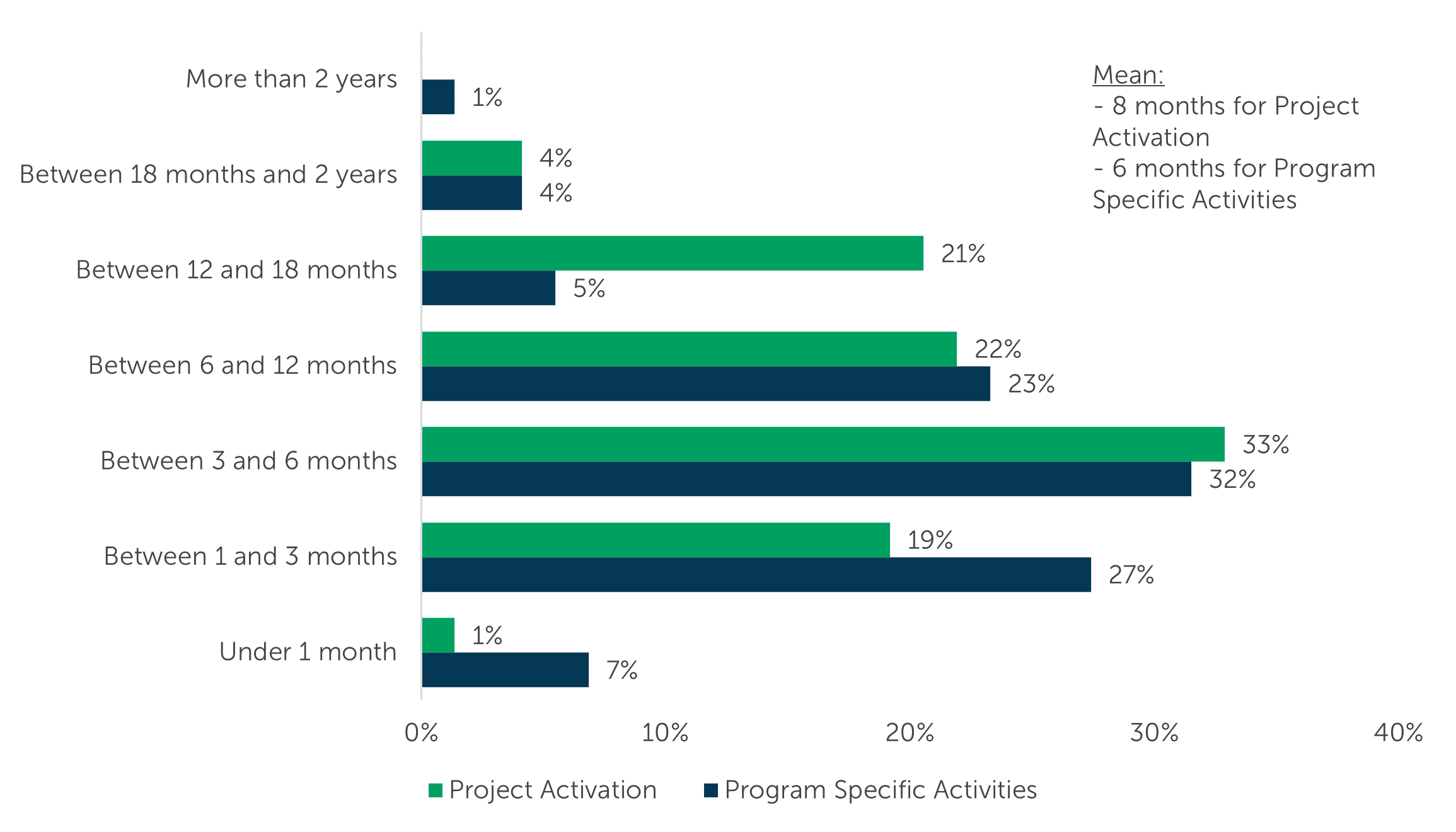
Most Important Gene Therapy CDMO Selection Attributes
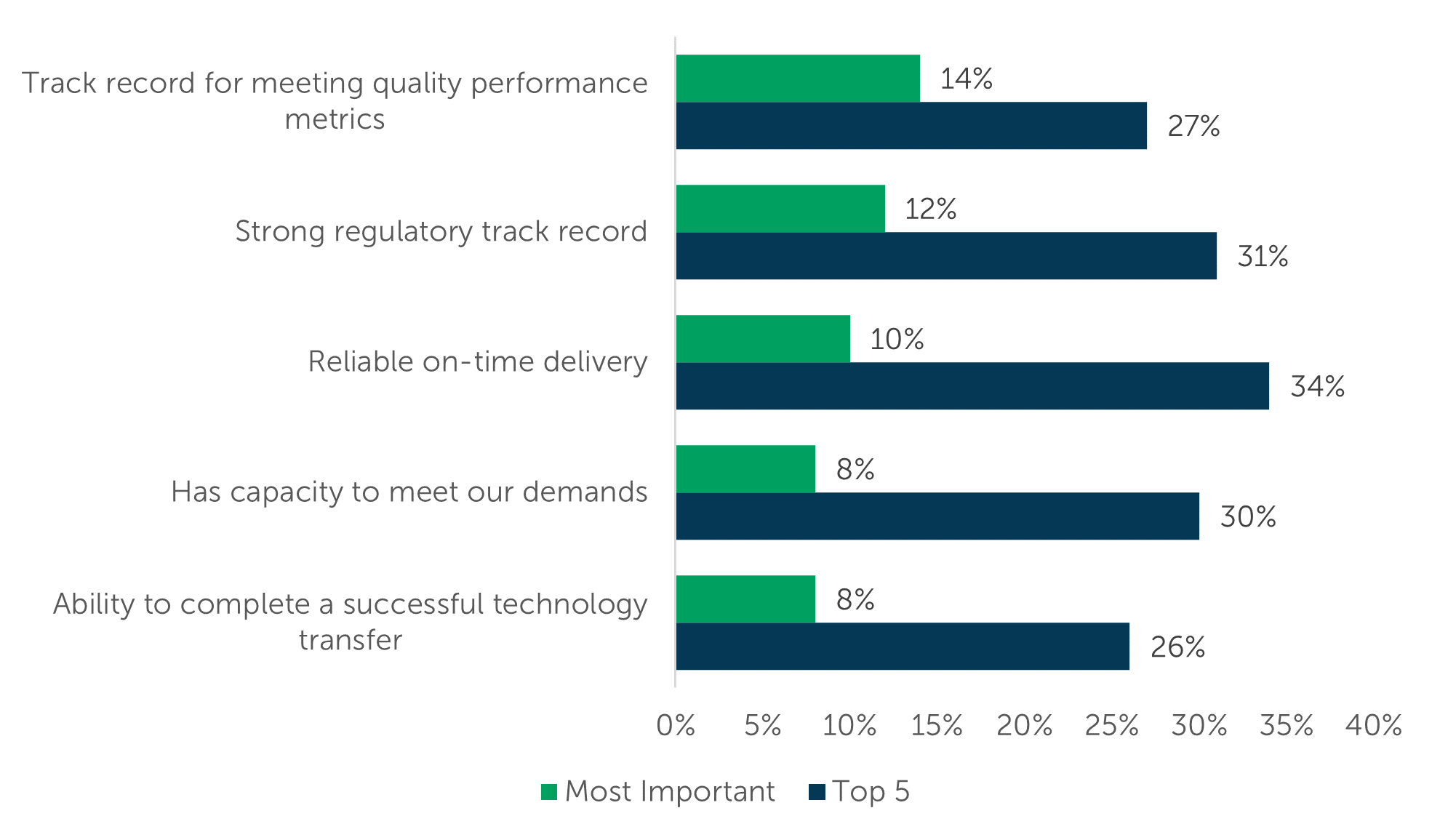
Survey participants were prompted to select their “Top 5” and single “Most Important” attribute(s) when choosing a manufacturer for their gene therapy projects. Reliable on-time delivery (34%), Strong Regulatory track record (31%), and Has capacity to meet our demands (30%) received the most “Top 5” votes.
Track record for meeting quality performance metrics (14%), Strong regulatory track record (12%), and Reliable on-time delivery (10%) made up the largest proportion of “Most Important” votes. The respondent group identified a total of 17 different selection metrics as ‘Most Important’ (with only an eleven percentage point difference between first and last place), highlighting the breadth of criteria sponsors use to evaluate potential providers. Gene therapy contract manufacturers can focus their external messaging on how well they perform on these most important attributes to stand out from competitors and to position themselves as the ideal outsourcing partner.
“Please review the following attributes and select the 5 most important to you when selecting a provider for gene therapy contract development and manufacturing services.” (n=77)
“Among the following attributes, please select the 1 most important to you when selecting a provider for gene therapy contract development and manufacturing services.” (n=77)
Gene Therapy CDMO Benchmarking
This report is the latest addition to our suite of CDMO benchmarking research and the first to focus exclusively on gene therapy manufacturing. Beyond information on project timelines and selection attributes, this report reveals experiential data from verified decision makers with outsourcing responsibility at biopharma companies. Drug innovators can use these data to compare their own outsourcing practices to their peers and assess manufacturers during the CDMO selection process. CDMOs can also benefit by understanding key selection attributes that innovators seek in a manufacturing partner and benchmark their performance to that of their competitors.
ISR is driven to help advance the drug development industry through reliable and actionable market data. Our Gene Therapy CDMO Benchmarking report offers a comprehensive analysis of outsourcing trends and practices for the benefit of all players in this rapidly growing space.
Primary market research data in this article were powered by the ISR Health Panel. Want to contribute to thought leadership pieces and help to make the pharma industry better? Join today.
Learn More 2023 Gene Therapy CDMO Benchmarking
1Lapteva et al., December 2020. Clinical Development of Gene Therapies: The First Three Decades and Counting – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7658574/