The Chemistry, Manufacturing, and Controls Function
In the dynamic world of biopharmaceuticals, small and emerging companies play a vital role in driving innovation and advancing groundbreaking therapies. While these companies often possess cutting-edge science and promising drug candidates, they may face unique challenges in navigating the complex regulatory landscape and bringing their products to market efficiently. This is where the Chemistry, Manufacturing and Controls (CMC) function emerges as a key component for success. CMC professionals ensure the effectiveness, safety, and highest quality of drugs, making their contribution indispensable.
Industry Standard Research (ISR) conducted interviews with 15 CMC professionals from pharmaceutical and biotechnology companies, 11 of whom work in small or emerging biopharma companies. These insightful discussions shed light on the critical role and function of CMC in drug development.
Understanding the CMC Function
Timely completion is crucial in the biopharmaceutical industry, and the CMC function plays a vital role in accelerating the path to market for small and emerging companies. By adopting efficient manufacturing strategies, optimizing processes, and equipping CMC staff with resources to effectively conduct their various tasks, companies can expedite the development and manufacturing of their drug candidates. A streamlined CMC function can reduce time-consuming iterations, enhance process understanding, and ultimately lead to faster regulatory submissions and approvals1.
CMC Involvement in Preclinical, Clinical, and Commercialization Phases
CMC involvement throughout the preclinical, clinical, and commercialization phases of product development varies by organization. By examining how the CMC function is established in virtual and small biopharma companies, ISR identified three common approaches pertaining to CMC involvement through clinical phases (see one approach in Figure 1).
Figure 1 – Approach C: CMC involvement and influence in the product development process
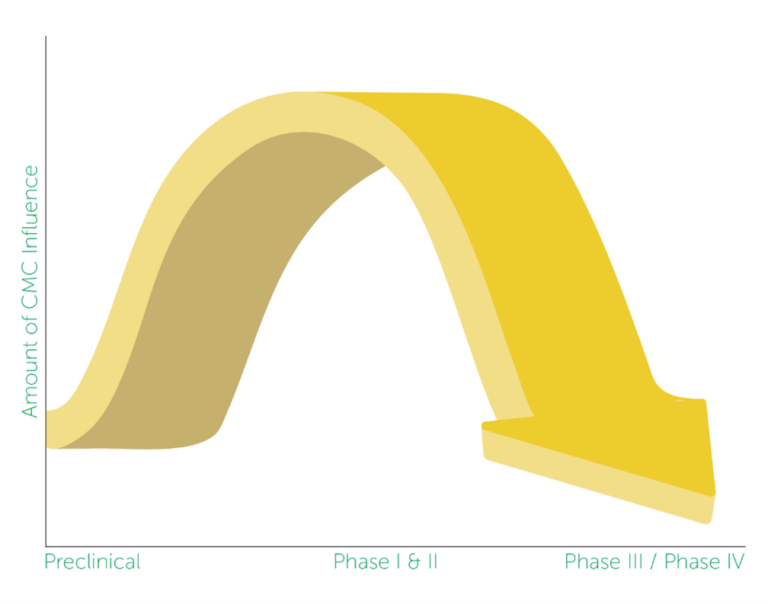
The CMC Function at Emerging Pharma report offers valuable insights regarding the amount of influence CMC provides throughout the clinical development process. By comparing their own approach to industry standards, peer companies can identify opportunities for improvement and ensure effective integration of CMC throughout each clinical phase. Furthermore, understanding these structures will provide valuable insights into industry-specific best practices.
Key Performance Indicators and Resource Allocation
As emerging biopharma seek success in drug development, measuring progress and allocating resources effectively are key elements. The approaches taken by different companies in measuring success within the CMC function are not as formalized or similar as you might expect. However, the underlying goal is the same: effectively complete tasks on the path to successful outcomes for new medicines.
Limited Budgets
Restricted budgets in the past year have posed challenges for CMC professionals. Hear verbatim how CMC professionals have advocated for increased funding, have ensured security of supplies, or otherwise found solutions to the challenges related to limited budget.
”“I want all venture capital people who invest in biotech companies to have a conversation with one or two CMC people and understand what goes into this process. Because I think the people who have the money and make the decisions have historically been too far away from this process, so they can just envision it as a black box.”
(Small Biopharma, Biologics, CMC Program Lead)
”“Most of our challenges are related to funding, getting the money, because making this stuff isn't all that complicated, but you still need to pay for it.”
(Small Biopharma, Small Molecules and Biologics, Director of CMC & Quality)
While 13 of the 15 interviewed indicated the funding they receive is appropriately allocated, they also expressed the converse: that additional funding would greatly enhance their ability to carry out their work more effectively. An efficient CMC function plays a crucial role in identifying cost-effective alternatives without compromising the quality of the products, thus ensuring long-term financial viability.
In small and emerging companies, it is not uncommon for CMC professionals to directly collaborate with the C-suite executives to secure funding. When a well-established CMC function is in place and there is open communication and understanding between the CMC team and the company’s C-suite, the company can successfully develop drugs while also maintaining cost efficiency, something at the forefront of emerging biopharma companies’ minds.
Discovering Best Practices in Chemistry, Manufacturing, and Controls
One of the key highlights of the CMC Function at Emerging Pharma report is the exploration of CMC-specific roles and responsibilities with a compilation of CMC best practices and recommendations. By understanding how successful CMC professionals operate, companies can optimize their approach to drug development. From employee attributes to outsourcing behaviors, this report unveils the best practices that contribute to CMC excellence.
Future Guidance for CMC
CMC is an integral role in biopharma, specifically in small and emerging biopharma companies. By exploring best practices, responsibilities, departmental structures, success metrics, resource allocation, and CMC involvement by clinical phase, the CMC Function at Emerging Pharma report equips companies with the knowledge needed to optimize their CMC operations. Leveraging your CMC Department’s expertise will enable companies to navigate drug development challenges with precision, ensuring the production of effective, safe, and high-quality therapies for patients in need.
Primary market research data in this article were powered by the ISR Health Panel. Want to contribute to thought leadership pieces and help to make the pharma industry better? Join today.
Learn More The CMC Function at Emerging Pharma
1Food and Drug Administration, https://www.accessdata.fda.gov/static/cvm/cormier/CMCsandGMPs.pptx