Evolving Perceptions and Practices in Sterile Injectable Outsourcing
Sterile injectable medicines provide significant advantages in terms of rapid and accurate drug delivery. Many newer medications cannot be produced as an oral dosage form for various reasons, making injectable delivery an effective means of treating numerous medical conditions and improving patient outcomes. Biopharmaceutical companies often entrust the production of sterile injectable drugs to Contract Development and Manufacturing Organizations (CDMOs), allowing them to leverage external expertise and state-of-the-art facilities to manufacture these complex drugs efficiently and safely.
To better understand how perceptions and practices evolve in this space, Industry Standard Research (ISR) surveys biopharmaceutical decision makers to uncover the latest outsourcing trends in sterile injectable manufacturing. This article will explore three iterations of our Sterile Injectable Drug Product Manufacturing Market Outlook (published in 2019, 2021, and 2023) for a look at how the experiences and perspectives of decision makers at biopharmaceutical companies have changed over time.
Shifts in Most Important Selection Attributes for CMOs
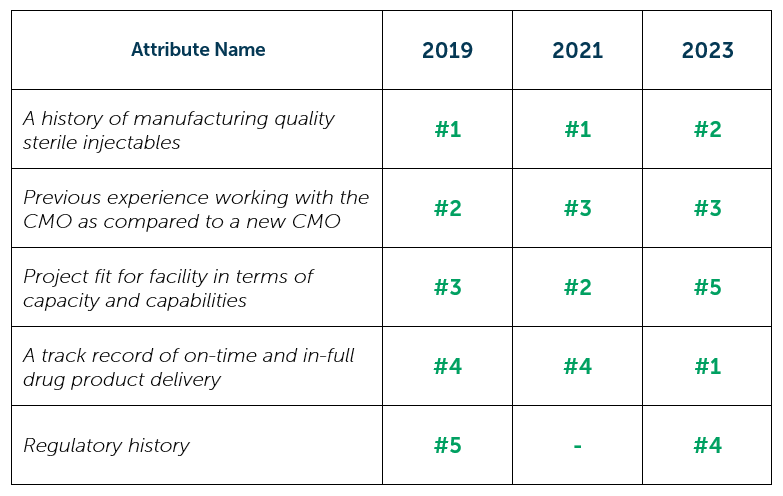
Looking at some of the most important CMO selection attributes, respondents have indicated that they seek a provider who is familiar and carries a good reputation. A history of manufacturing quality sterile injectables held the number 1 spot for 2019 and 2021 but was unseated by A track record of on-time and in-full drug product delivery for 2023’s report. Previous experience working with the CMO as compared to a new CMO remains in the top 3 year-over-year, whereas Project fit for facility in terms of capacity and capabilities dropped to the bottom of the top 5 for 2023. Regulatory history falls out of the top 5 for 2021, and while it is not fully captured below, it is worth noting that around 40% of respondents consistently place this attribute in their top 5.
Sterile Injectable CMO Selection Attributes 2019 – 2023
“Thinking of the service provider attributes that you value the most when selecting a CMO for outsourced sterile injectable drug product manufacturing, which attributes do you find important? Select five.” (n=varies)
“From these attributes, which one attribute do you value most when selecting a CMO for outsourced sterile injectable drug product manufacturing? Select only one.” (n=varies)
Shifts in Greatest Challenges Outsourcing Sterile Injectable Manufacturing
Drug innovator companies face many challenges in terms of sterile injectable manufacturing. Of recent note, biopharmaceutical giant Pfizer has been working to provide relief and repair damage caused by a tornado at its facility in Rocky Mount, NC, one of the largest sterile injectable drug product manufacturing sites in the world. As the company explores alternative manufacturing locations and works to restart production, it is possible that the biopharma industry will experience additional capacity constraints on sterile injectable manufacturing until Pfizer’s Rocky Mount facility is back to running at full capacity.
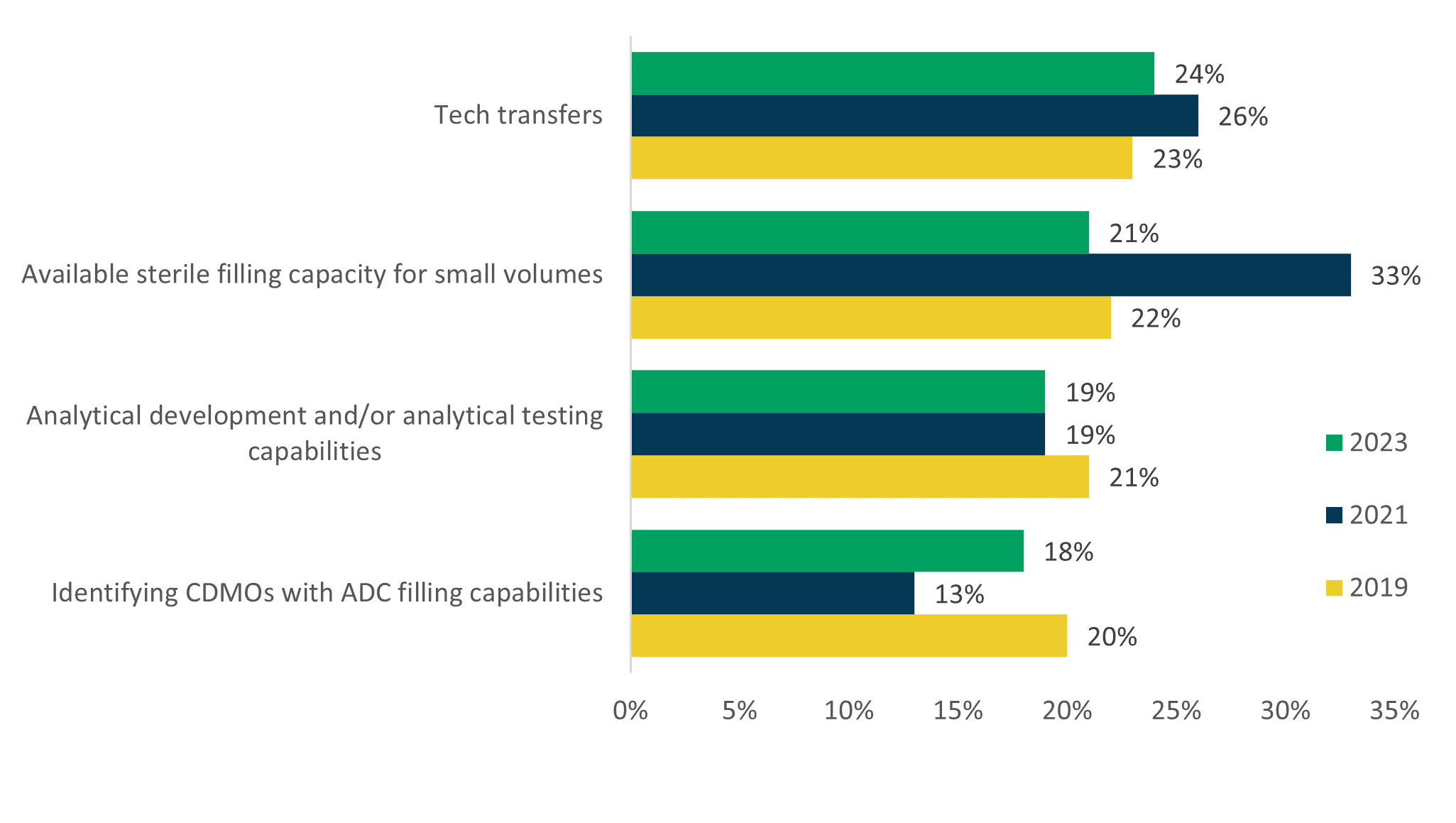
In our research, there is variability in the verbatim explanations provided by respondents regarding the typical challenges they face when outsourcing these projects, but the data show strong similarities year-over-year. Tech transfers is arguably the most cited challenge, but Available sterile filling capacity for small volumes experiences a stark jump in 2021 and was a standout in the list for that year at 33%. Identifying CDMOs with ADC filling capabilities also stands out due to a significant drop between 2019 and 2021 but has risen to prior levels in 2023. The challenge of Analytical development and/or analytical testing capabilities holds steady across the three years.
Greatest Challenges Outsourcing Sterile Injectable Manufacturing
“What is the biggest challenge your company faces when outsourcing sterile injectable drug product manufacturing to CDMOs/CMOs?” (2023 n=119; 2021 n=102; 2019 n=101)
Inform Your Outsourcing Strategies with Market Research
Sterile injectable drugs need to be produced and handled under strict conditions to prevent contamination and ensure patient safety. By outsourcing this important aspect of drug manufacturing, innovators can focus on R&D, other core competencies, and accelerate new product development. To manage these outsourced projects effectively, it is critical for players in this space to have an understanding of development stage and marketed drug product volume, increased use of manufacturers, and other changing outsourcing behaviors. ISR seeks to provide actionable market research data to inform drug developers as well as manufacturers of the latest industry trends to enable both companies to make the best decisions possible for their businesses.
Primary market research data in this article were powered by the ISR Health Panel. Want to contribute to thought leadership pieces and help to make the pharma industry better? Join today.




One Comment