
Introduction
My name is Brian and I’m the VP of Clinical Operations at a global CRO. My job is to ensure our clinical operations team is prepared to meet the demands of our clients. I am also part of a task force that is charged with staying current with market trends and recommending which capabilities should be added to our service offerings.
Importance of Understanding Client’s Needs and Expectations
To be successful at my job, I need to understand our clients’ current and future needs so we can ensure our capabilities and capacity are in line with the study types and volume they will request. I need a good understanding of the qualities sponsors are requiring in their CROs, where their studies are taking place, and what challenges they’re experiencing so I can best position my company to meet the needs of our potential customers.
Phase I Market Outlook Report
ISR’s Phase I Market Outlook (2022-2026) report provides clinical research organizations with an overview of the Phase I clinical development space to explore current market dynamics and inform strategic planning. For this report, 125 Phase I decision-makers were surveyed to share their insights into where the Phase I market is now and what changes they expect over the next four years.
Key Information for Service Providers
CROs can utilize the findings in this report to compare their capabilities and capacity to what sponsors indicate will be needed in the near future. Readers like me will gain a deeper understanding of the key decision-makers within sponsor organizations, learn the criteria sponsors use to select service providers, and uncover how the top criteria differ between simple and complex Phase I studies.
Understand the Phase I Market to Win Business
To capture the attention of drug developers and showcase how your CRO is the best fit for a clinical trial in this space, it’s critical to understand the evolution of trends in the Phase I market (e.g., diagnostic development, challenge trials, changes in study complexity, geographic expansion, pandemic-related changes expected to remain in place after the pandemic has subsided, etc.). ISR’s 4-year examination of the industry’s Phase I market dynamics includes respondent rationale for predicted changes, granting service providers valuable insight into sponsor preferences and practices.
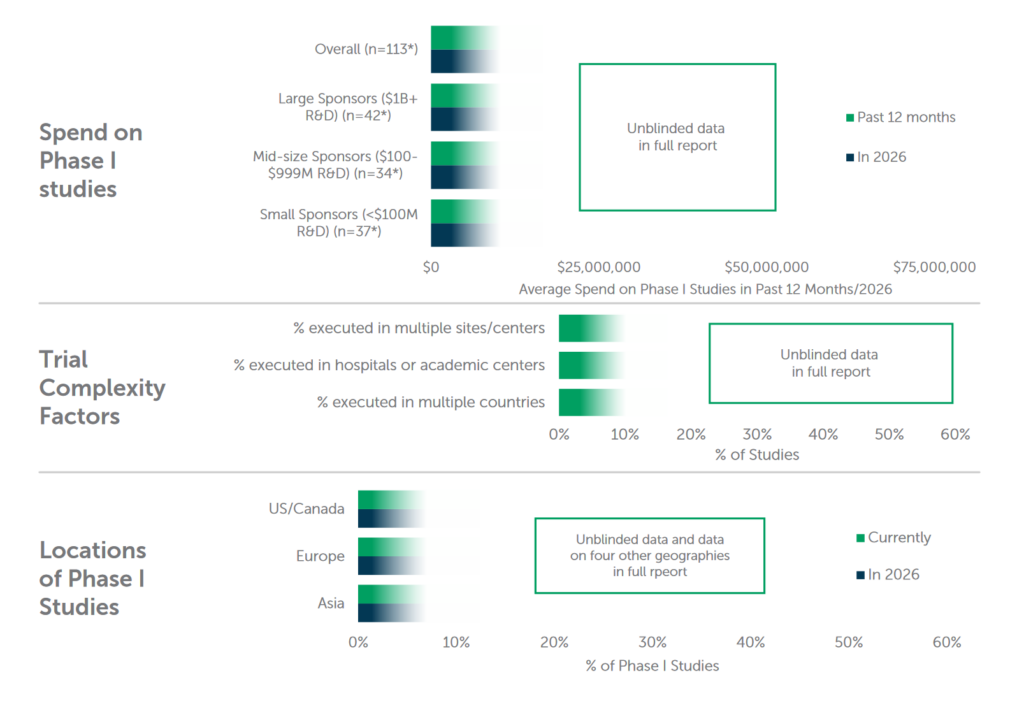
Here are a few data points that I found interesting in this report from ISR:
Learn more about how this report can help you understand current Phase I market trends, anticipate drug sponsor’s needs, and position your company as the ideal service provider.





