Patient-Reported Data in Clinical Trials
Patient-reported data is a cornerstone of clinical trial research, and thankfully bulky paper records are on their way to becoming a thing of the past. The recent advent of smart devices, in concert with an industry shift toward decentralized trials, has revolutionized the collection, analysis, and utilization of these data. Clinical technology service providers that offer eCOA/ePRO systems (Electronic Clinical Outcome Assessment/Electronic Patient-Reported Outcome) must ensure their platforms provide accurate, real-time data and can integrate with other clinical technology, streamlining relevant processes to make for faster and more efficient trials. These service providers must work diligently to ensure their software offers a good user experience for patients and clinical staff alike.
In order to delve deeper and support the platform providers, CROs, and pharmaceutical sponsors driving innovation in the eCOA/ePRO space, Industry Standard Research (ISR) recently conducted a survey of 119 experienced outsourcers to collect their insights on eCOA/ePRO platforms across 24 key attributes. Our eCOA/ePRO Benchmarking and Market Dynamics (5th Ed.) report is designed to inform readers of the latest trends in this space, allow providers to compare their performance to that of their competition, and provide sponsors and CROs with feedback from real customers to inform their outsourcing decisions. Survey participants provided valuable insights on the future of integration with other technologies including sensors and wearables, supporting data collection with the BYOD (bring-your-own-device) approach, and accommodating the new era of decentralized trials.
eCOA/ePRO Integration, Use of Sensors and Wearables
Not surprisingly, one of the most critical features that sponsor and CRO organizations seek in an eCOA/ePRO solution is the ability to integrate well with other eClinical technologies. This finding was consistent throughout the research; integration is the leading selection driver, tops the list of attributes that impact trial success, and is among the most desired improvements to eCOA/ePRO systems.
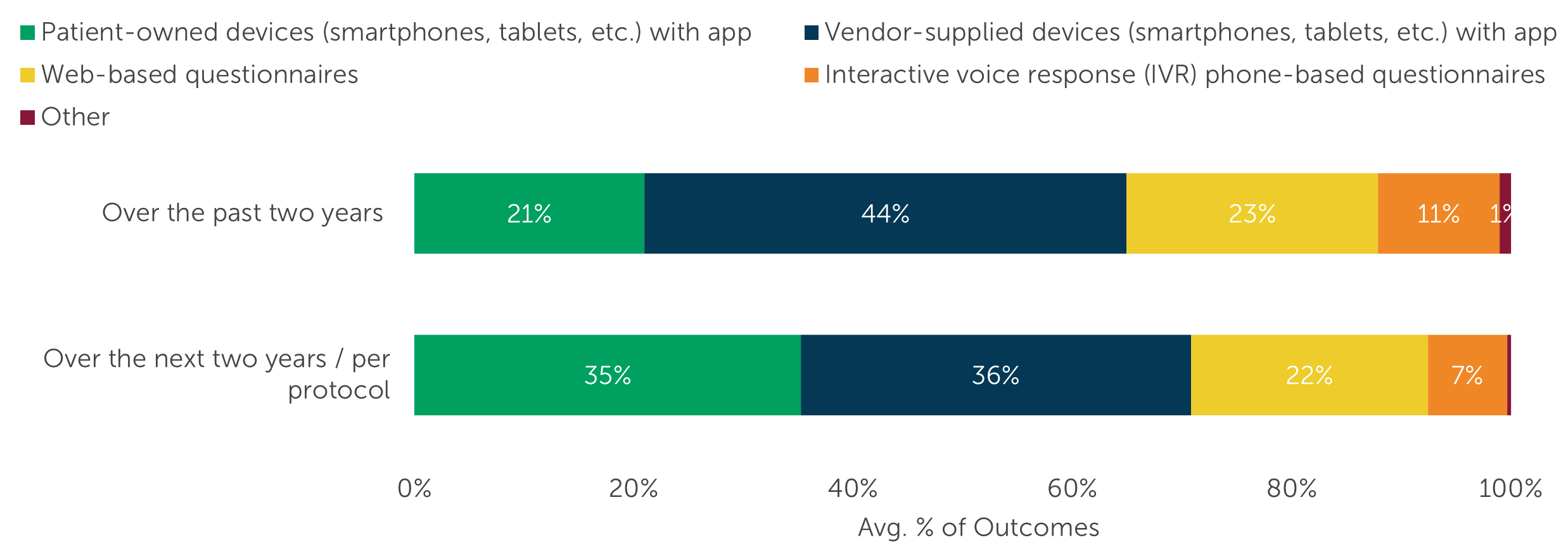
However, while integration with EDC, IRT, and other technologies is critical, eCOA/ePRO systems in particular are also under pressure to support integration with a variety of patient-facing data sources, such as the BYOD approach for patient questionnaires. In our 2023 research, respondents predicted that the proportion of outcomes reported via Patient-owned devices (smartphones, tablets, etc.) with [an] app would increase from an average of 21% to 35% over the next two years.
“Over the past two years, what percent of reported outcomes on your clinical trials have come from the following sources? What will the percentage be for each source over the next two years? Your best estimates are fine, but values must sum to 100.” (n=119)
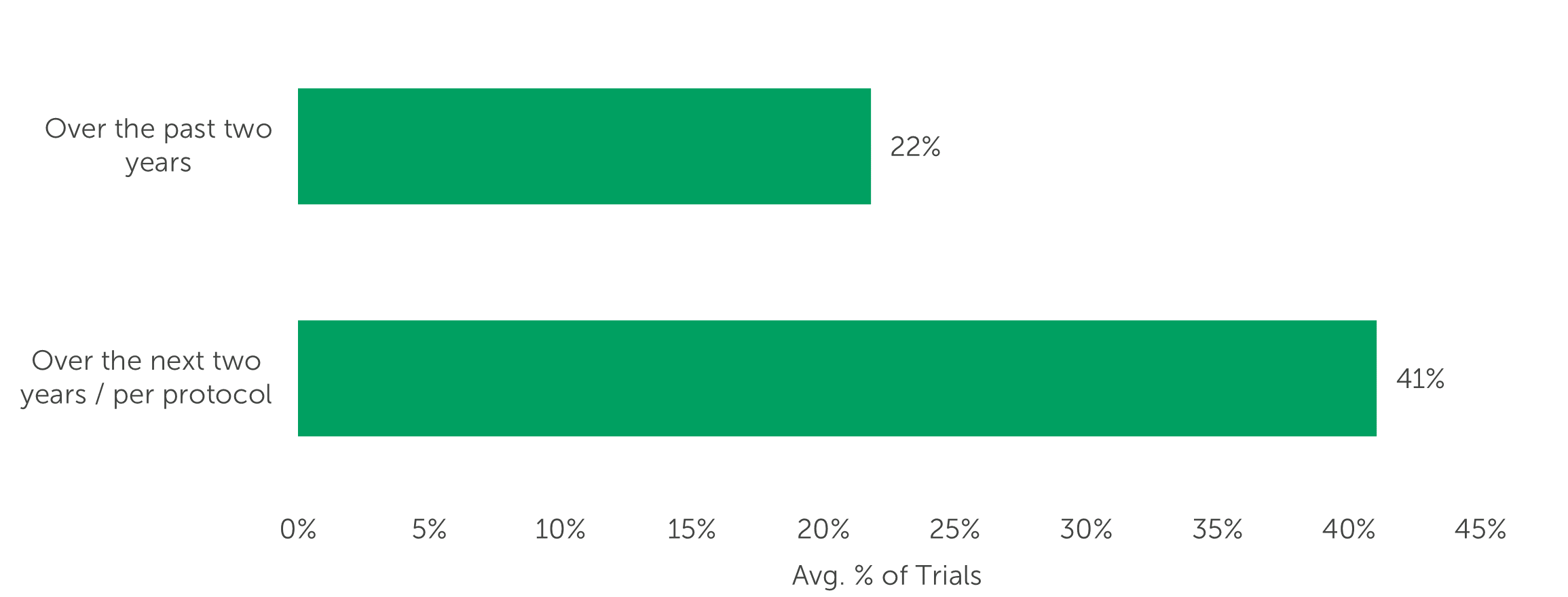
Furthermore, respondents ranked eCOA/ePRO Integration with sensors and wearable devices second highest in a list of attributes expected to become more important when selecting an eCOA/ePRO provider over the next 12 months. Respondents predict that the number of trials that collect patient reported outcomes and also use sensors and/or wearables will increase from an average of one-fifth (22%) of trials over the past two years to two-fifths (41%) of trials over the next two years.
“Over the past two years, what percent of your clinical trials that collected patient reported outcomes also used sensors and/or wearables to collect objective measurements directly from patients (e.g., glucose meter, spirometer, activity meter)? What will the percentage be over the next two years? Your best estimate is fine.” (n=119)
Unmet eCOA/ePRO Needs in Decentralized Trials
Another factor driving significant change in the eCOA/ePRO ecosystem is the growing demand for decentralized trials. Although decentralized trials require extensive technology infrastructure to support more local patient care, remote monitoring, and remote data capture, we at ISR consistently hear from industry experts that too many “bells and whistles” on decentralized technologies can be overly burdensome for sites and patients.
When we asked respondents to describe their unmet needs related to eCOA/ePRO usage in decentralized trials, one-fifth (20%) mentioned they’d like to see improved integration between eCOA/ePRO systems and sensors/wearables, EDC, or eConsent systems. In keeping with the importance of patient centricity and alleviating patient burden, respondents also mentioned that they’d like eCOA/ePRO systems to encourage patient compliance and patient engagement (18%) and offer patient training (14%).
“What are your unmet needs related to eCOA/ePRO usage in decentralized trials?” (n=95, only asked of respondents with experience with decentralized trials and some level of unmet needs. Open-ended responses have been themed)
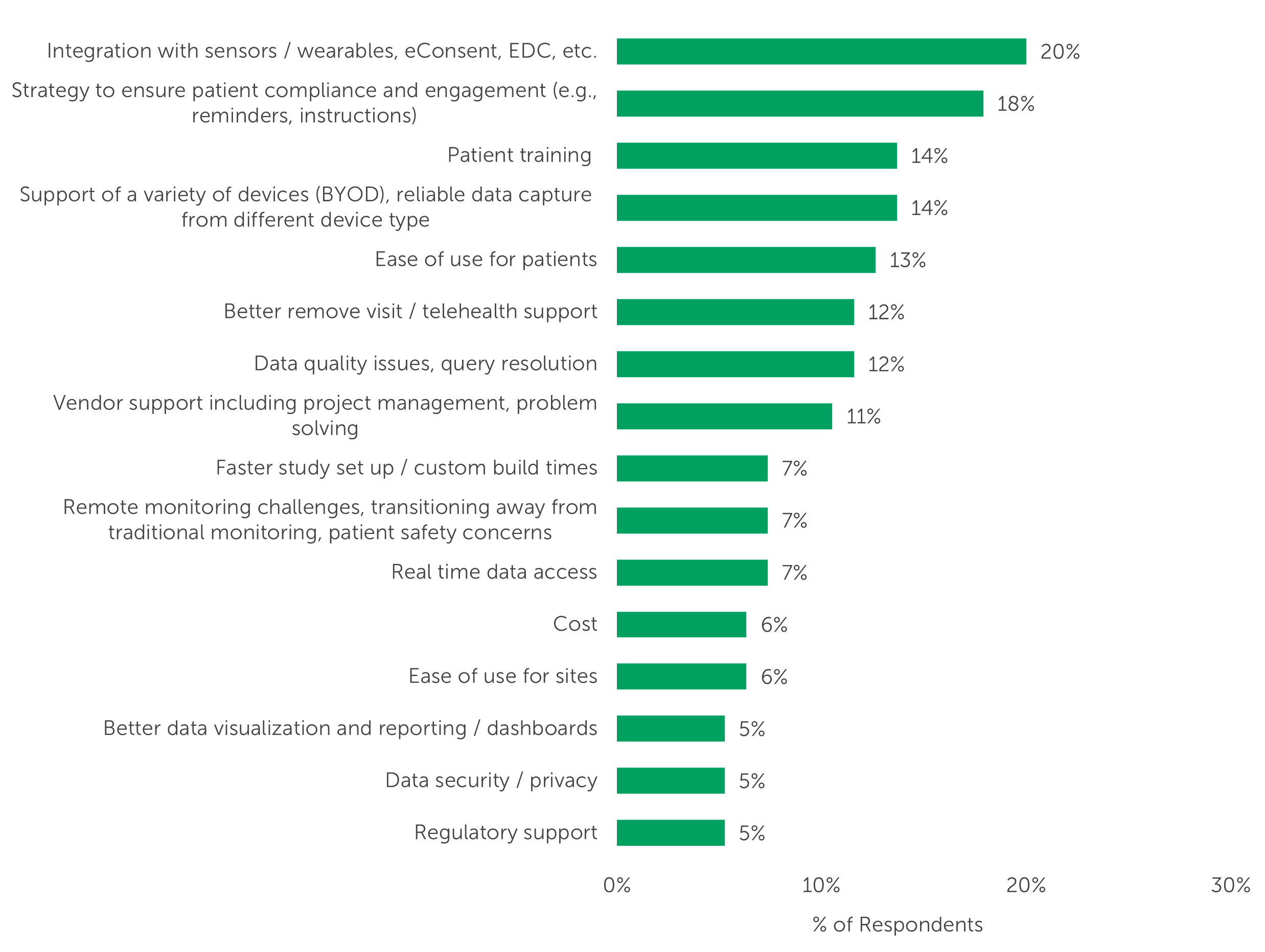
*Only themes mentioned by ≥5% of respondents shown. See Study Data for full data set.
eCOA/ePRO Benchmarking and Market Dynamics (5th Ed.)
Beyond information on patient device use and decentralized trial needs, our latest eCOA/ePRO report offers readers the chance to learn what service attributes and capabilities sponsors seek in a provider, as well as how the outsourcing community evaluates and maintains relationships with these providers. eCOA/ePRO providers can understand how their brand is perceived in the market as well as read verbatim responses regarding why respondents prefer specific solutions and proposed improvements that customers would like to see in the future.
ISR is driven to help advance the drug development industry through reliable and actionable market data. Our eCOA/ePRO Benchmarking and Market Dynamics (5th Ed.) report offers a comprehensive analysis of outsourcing trends and practices for the benefit of all players in the clinical development space.
Primary market research data in this article were powered by the ISR Health Panel. Want to contribute to thought leadership pieces and help to make the pharma industry better? Join today.




