Biopharmaceutical sponsor considerations when selecting a CDMO may include timelines, cost, regulatory requirements, capacity, quality, risks, and previous experience. To help sponsors better understand and prioritize development and manufacturing elements, ISR conducts four annual, manufacturing-specific benchmark studies that ask professionals in the biopharmaceutical sphere about their experiences with CDMOs.
Respondents evaluate suppliers they have worked with in the past 18 months on 23 performance metrics, which include attributes related to company strengths, capabilities, staff characteristics, services, and more. Performance benchmarking (a market research tool) can help to make CDMO selection less arduous.
Top Attributes
Every year, ISR asks respondents which attributes are among their “Top Five” in CDMO selection, which attribute is “Most Important,” and which attribute is gaining in importance across the four manufacturing areas (i.e., biologic API, biologic drug product, small molecule API, and small molecule drug product). In 2022, the most important attributes varied, but the attribute gaining in importance was shared across all four benchmarks (Fig. 1).
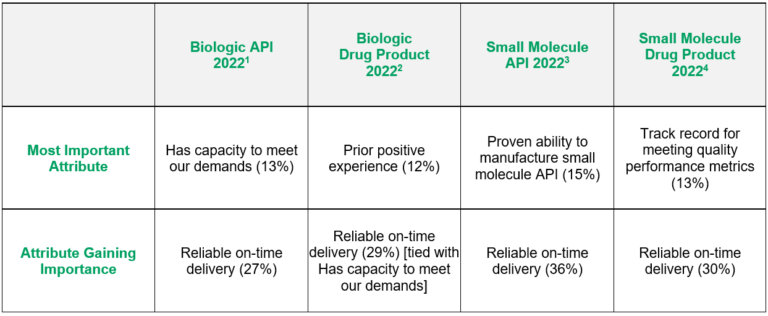
Fig. 1
While biopharmaceutical professionals selected four different attributes as “Most Important,” across the four manufacturing areas, it is not hard to see how these attributes correspond with one another. ISR sees this trend every year, with the “Most Important” trait varying, but the proportions between attributes not changing significantly. In other words, it is not likely that just one attribute is selected as “Most Important” by a large margin, nor do we see any attributes omitted.
In the 2022 Small Molecule Drug Product CDMO Benchmarking,4 13% of respondents chose a CDMO’s track record for meeting quality performance metrics as the most important selection driver. In 2021, the “Most Important” attributes comprised a three-way tie: Low cost, Prior positive experience, and Ability to smoothly scale up manufacturing. While each of those criteria dropped fell in ranking in 2022, each remained in the top 10. On the other hand, all benchmarks indicated that respondents see Reliable on-time delivery as the attribute gaining importance.
Priorities in CDMO Selection
So how have those trends evolved over the past three years (2020 to 2022)? Three areas stand out as the highest priorities for biopharma sponsors when choosing a CDMO: low cost/affordability, capacity to meet demands and/or the ability to manufacture the specific drug component, and timeliness. (Fig. 2).
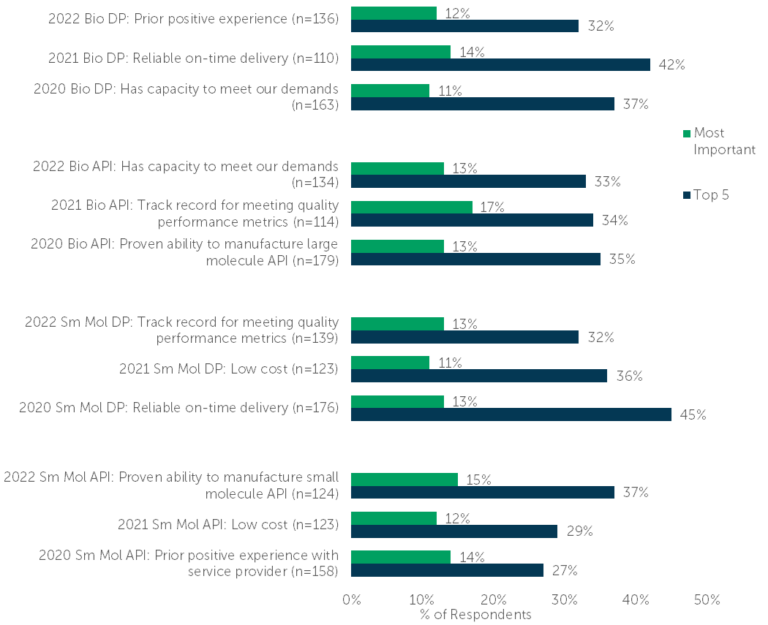
Fig. 2 – “Please review the following attributes and select the [five/one] most important to you when selecting a provider for [biologic API/biologic drug product/small molecule API/small molecule drug product] manufacturing services.” (n=listed with study, as it varied)
All things considered, the past three years have been unique, tumultuous, and marked by unusual challenges. The biopharma industry has been — and, in many cases, continues to be — impacted by supply chain issues, unemployment, people leaving their jobs, the general disruption of work (e.g., shifting to remote work and back again), plus any number of other factors. Whether on-time delivery or prior positive experience was stated as most important by benchmarking respondents, the end message is clear: biopharmaceutical professionals value an affordable, on-time, quality result.
CDMO selection is not an easy process. ISR aims, through our benchmarking reports asking biopharmaceutical professionals about their recent experiences, to alleviate some of that burden. Click below to explore the most recent benchmarking studies and to learn more about how market research tools can serve your organization.