I bet you can’t say “cell & gene therapies” three times fast. And by the time you do, you’ll have missed another article on your newsfeed discussing their intricacies and the headaches they can cause. Here’s one from Industry Standard Research: read on for a closer look at the findings in ISR’s Cell & Gene Therapies Market Outlook report, which offers some insight into this growing field for innovators and manufactures alike.
These data were collected in Q3, 2021 and include responses from 101 outsourcing decision-makers at biopharma companies who had responsibility for cell and/or gene therapies within the past 18 months. Many of these respondents are also members of the ISR Health Panel, a community of industry professionals that provides insight into drug development trends and outsourcing behavior.
The perceptions captured in this report make no bones about it: there is a demand for expertise and scalability from biopharma innovators. The newness of these therapies dictates the need to outsource – sponsors are in the process of building up facilities, but they are not online yet, or sponsors are waiting until the therapy is closer to commercialization to invest in facilities. Currently 44% outsource most or all of their activities to CDMOs and that number is expected to drop by half over the next 5 years as a result (see chart below). Manufacturers can use this report to compare how well their offerings match what sponsors intend to outsource in the near future, as well as gain insight into the selection metrics that will help them win the outsourced business.
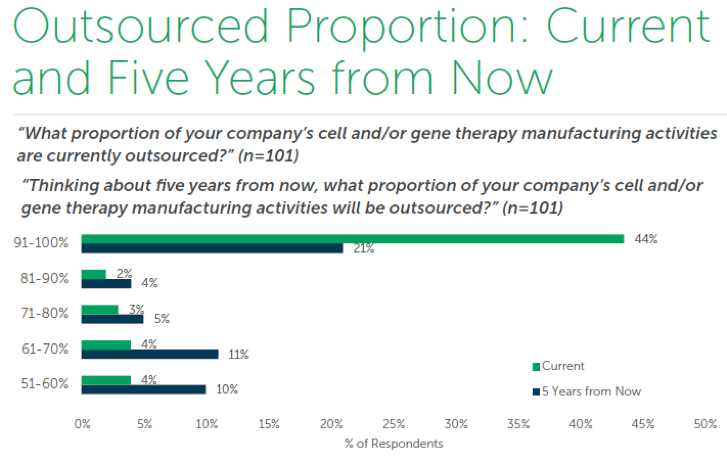
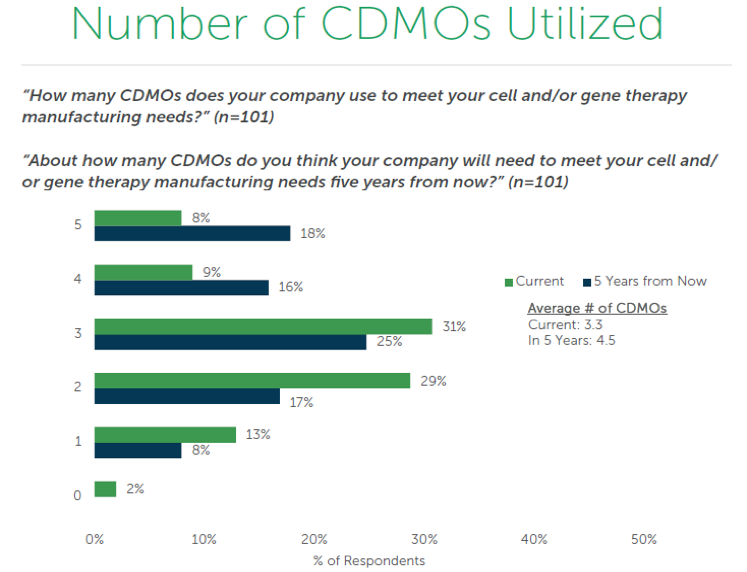
Therapy innovators can benefit from this report by using it to anticipate issues with logistics as they seek to scale up production. Once a therapy has been approved, there is a higher demand to manufacture the components which will potentially require more CDMOs to keep pace. This is especially true once a therapy is approved in more regions; finding a local provider becomes vital to production because cell & gene therapies are difficult to transport and do not have a long shelf life, often mere hours. This trend is highlighted in the next chart that displays how many CDMOs are used by innovators to meet their cell & gene therapy manufacturing needs. The current average is about 3 and that is expected to increase by 1 in the next 5 years.
“Uncertainty” is a word that has loomed over everyone for the last 2 years and the logistics challenges facing cell & gene therapies are also plagued by this diction. ISR offers to alleviate some of that ambiguity and give companies operating in this space a chance to regroup and strategize for these future shifts with the Cell & Gene Therapies Market Outlook.
Register an account for access to ISR’s collection of informative free resources such a whitepapers, infographics, and industry statistics.
Feel free to email ISR’s point of contact for syndicated research, Brandon Allison (BrandonA@isrreports.com), with any questions or for additional information.


2 Comments