Electronic data capture (EDC) systems have been a part of the clinical trial ecosystem for several decades and are now one of the most mature clinical technologies on the market. However, there are constant pressures to evolve to support decentralized trials in a changing environment for data collection and data quality. In our recent survey, EDC Market Dynamics and Service Provider Benchmarking (5th edition), ISR asked EDC users at sponsor organizations and CROs about the trends they expect to see over the next two years. Survey participants provided valuable suggestions for how EDC systems could keep up with decentralized clinical trials and expressed the importance of direct data capture.
Decentralized Trial Support
For the purposes of this research, we described decentralized trials as trials in which patients participate outside of a traditional clinical site (e.g., from home) for some visits/activities rather than exclusively in a clinical setting. When asked how well current EDC systems have met their needs to successfully execute a decentralized trial, roughly 9 out of 10 survey respondents (89%) indicated that their EDC systems did not completely meet their needs.
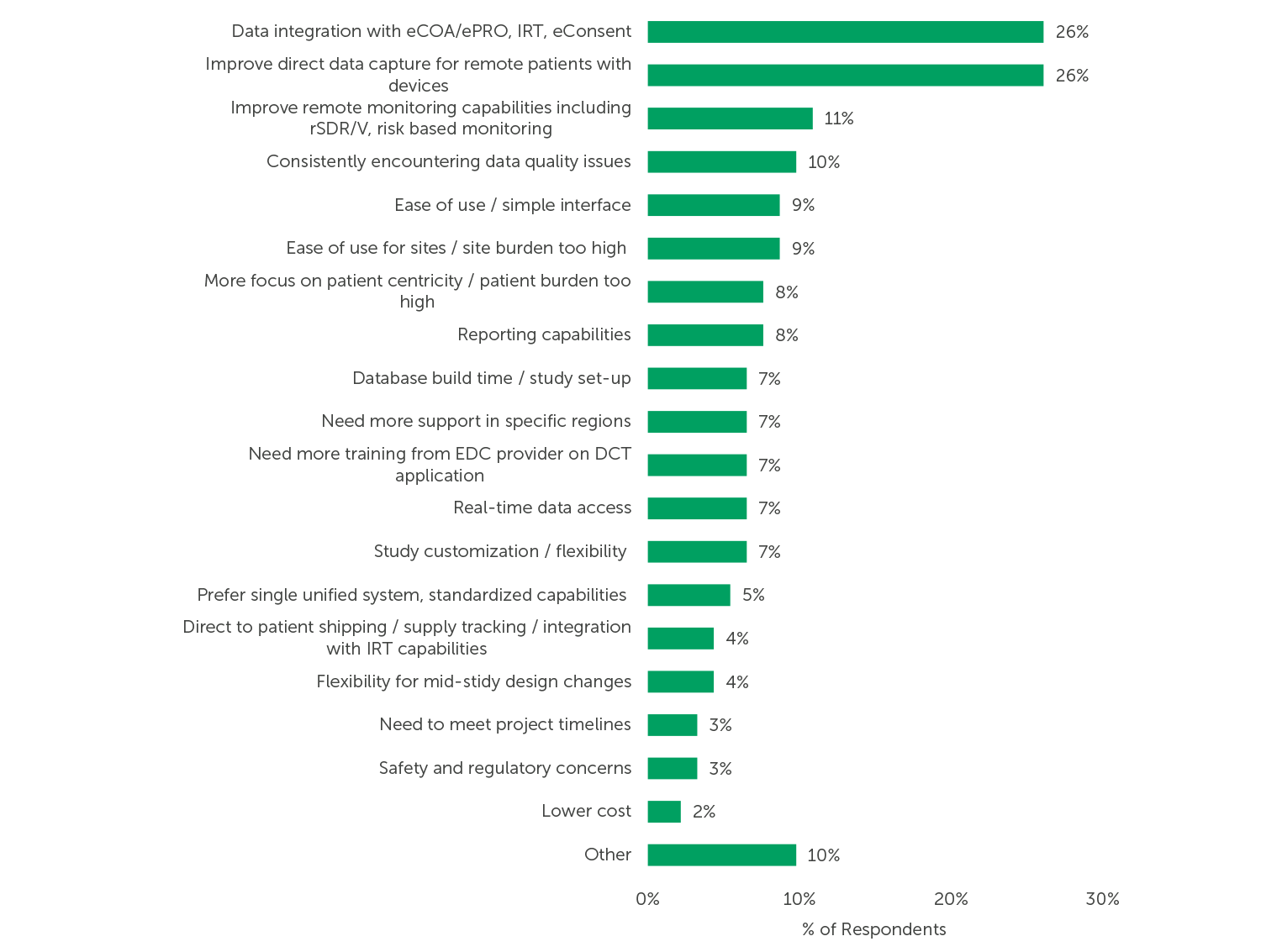
In describing their unmet needs related to EDC use in decentralized trials, approximately one-quarter of respondents mentioned that they would like to see better data integration with eCOA/ePRO, IRT, and eConsent systems (26%) and improved direct data capture from remote patients (26%). Respondents also suggested improving remote monitoring capabilities (11%), data quality (10%), and ease of use for sites (9%). As described in the verbatim responses below, EDC users value flexibility and improvements that reduce the burden on patients and sites.
”Flexibility to have the possibility to record onsite visits and remote visits. Better dynamics to accommodate different situations and avoid missing data. Better accommodation for risk-based monitoring options. Better integration of external data and easier validation/reconciliation processes.
Small Sponsor, Clinical Operations
”Better development of remote data entry options (e.g., smartphones, tablets) and integration with eCOA and wearables.
Large Sponsor, Clinical Operations
”Input of data — site staff need more training. Site staff need more incentive to input data, e.g., easy interface.
Mid size Sponsor, Medical Director
Fig. 1 (n=92) “What are your unmet needs related to EDC usage in decentralized trials?” (n=92, only asked of respondents with experience with decentralized trials and some level of unmet needs, open-ended responses have been themed)
*Additional suggestions available in the report for purchase.
Direct Data Capture
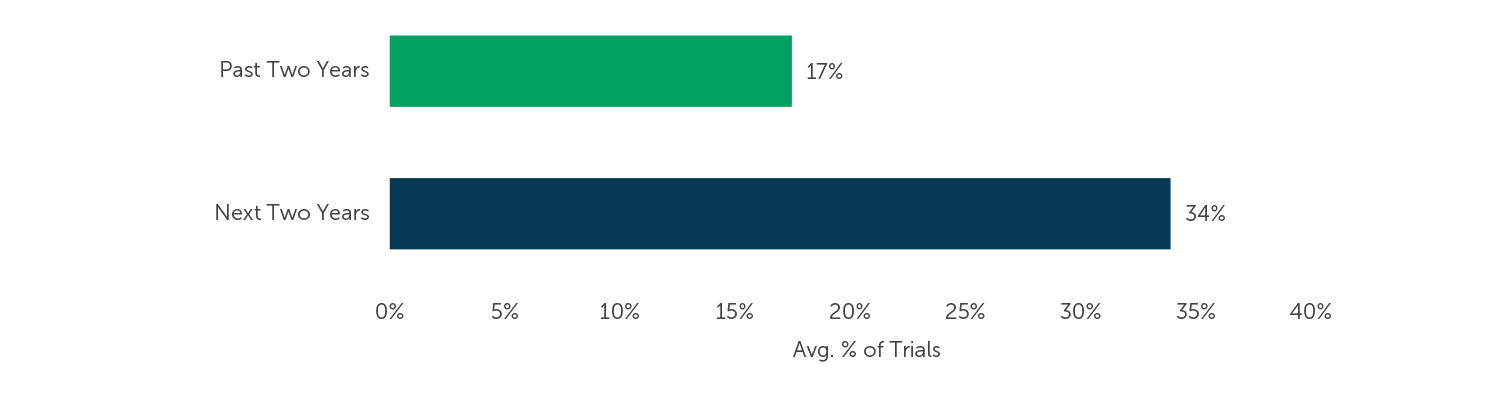
Industry experts and survey participants alike are excited about direct data capture, a method of capturing electronic data directly from patient devices and other systems. When asked about the proportion of clinical trials that collect patient data transmitted from sensors and/or wearables directly into the EDC system, respondents predicted a significant increase, from an average of 17% of their EDC trials over the last two years to an average of 34% over the next two years.
Fig. 2 (n=114) “What percentage of your EDC trials collected and will collect patient data transmitted from sensors and/or wearables (e.g., glucose meter, spirometer, activity meter) directly into the EDC system?”
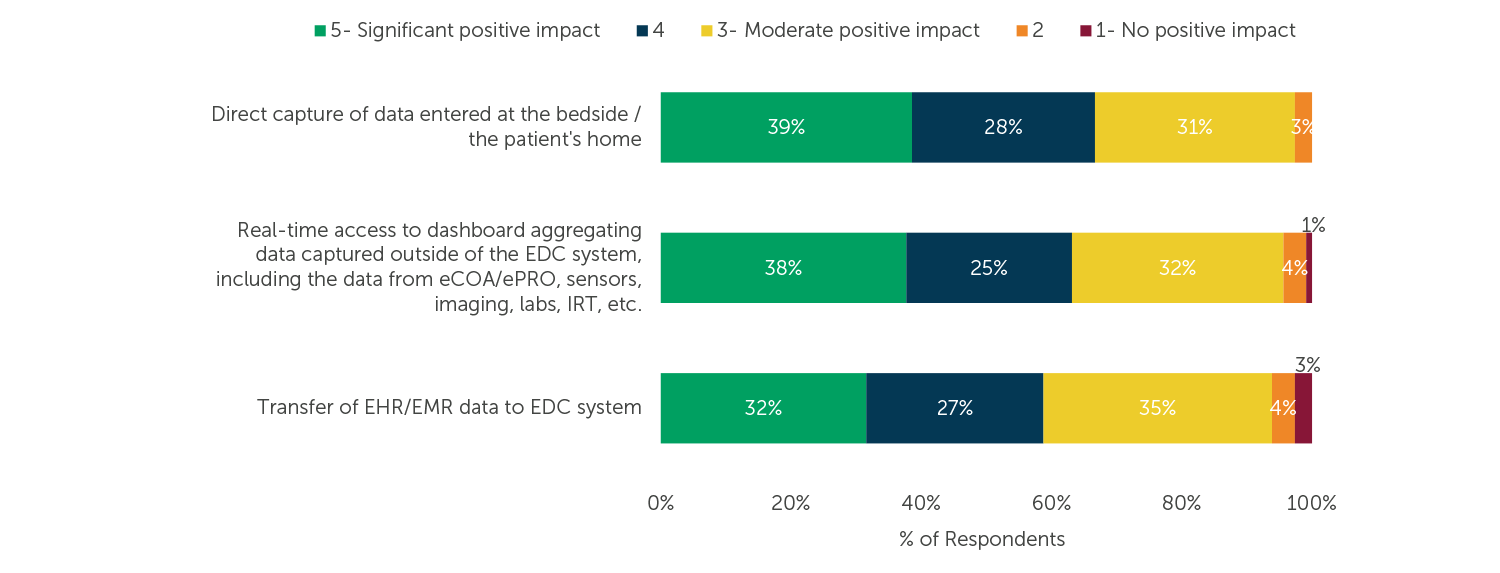
The direct data capture approach eliminates the need to download data from patient devices or transcribe documents; it also enables real-time access to data captured remotely. Underscoring the importance of patient centricity and ease of use for sites, the majority of respondents agreed that each of the trends described in the chart below would have a positive impact on the conduct of their clinical trials over the next two years.
Fig. 3 (n=114) “How would the following EDC trends related to reducing site burden and improving patient oversight impact your clinical trials over the next two years? Please evaluate each trend on a scale of 1 to 5 where: 1=this would have no positive impact on the conduct of our trials and 5=this would have a significant positive impact on the conduct of our trials.”
*Additional suggestions available in the report for purchase.
For clinical technology providers looking to differentiate their offerings, it’s important to note that EDC users will value capabilities related to real-time data integration. Looking to other industries, consumers of cloud-based technologies take for granted the seamless and instant integration of data; between our smartphones and other devices, on our favorite online shopping sites, on our business platforms that automate day-to-day processes and track data, and the list goes on. Therefore, it’s not surprising that sponsors and CROs expect the same of clinical trial technology providers.
In our research on various clinical trial software systems, we at ISR consistently hear that waiting for data to be communicated to other systems and dealing with data reconciliation are major issues. As seen in Figure 3, enabling clinical trial site staff to enter data from a patient’s bedside or remotely at their homes and push data from electronic health records to an EDC system is likely to have a very positive impact on patient outcomes. Additionally, respondents expect the data entered in patient diaries, captured on biometric devices/sensors, gathered in labs, and managed by drug supply software to be available in real-time to facilitate faster clinical trials.
Although the road to developing and improving these capabilities is anything but easy, we anticipate that the next generation of EDC will focus on decentralized trials and direct data capture. Early adopters rejoice and luddites beware; updates to the traditional EDC systems are on the way.




2 Comments