Decentralized clinical trials (DCTs) are studies in which some or all trial-related activities are conducted at a location separate from the investigators’ primary site. DCTs’ numerous benefits include convenience and flexibility for patients, greater opportunities for patient recruitment, increased patient diversity, and the potential to decrease timelines and cost. However, as the necessity that drove DCT use (i.e., the COVID-19 pandemic[i]) wanes, how have biopharmaceutical sponsors’ opinions of DCTs — their utility, affordability, etc. — evolved?
Industry Standard Research (ISR) surveyed 114 individuals whose biopharmaceutical companies have leveraged DCTs within the past year to understand their experiences and to form expectations for how DCTs may be used in the future. Though not all respondents to our Decentralized Clinical Trials Market Outlook[ii] reported a smooth experience, nearly 60% came away with a positive overall impression of DCTs, while only 9% reported a negative impression. Even with some aspects regarded as challenging, respondents do not seem to consider decentralized trials as a temporary solution to be discarded. In fact, 80% of respondents expect the DCT model to be used more frequently than the traditional trial model over the next three years.
Areas of Investment
Another indication of continued DCT use is sponsors’ investment in a wide range of decentralization-related areas. Per ISR’s 2022 survey, the highest percentage of respondents (44%) selected “establishing partnerships with eClinical technology providers” as an investment area. Respondents disclosed investment in a host of other areas, as well (Fig. 1).
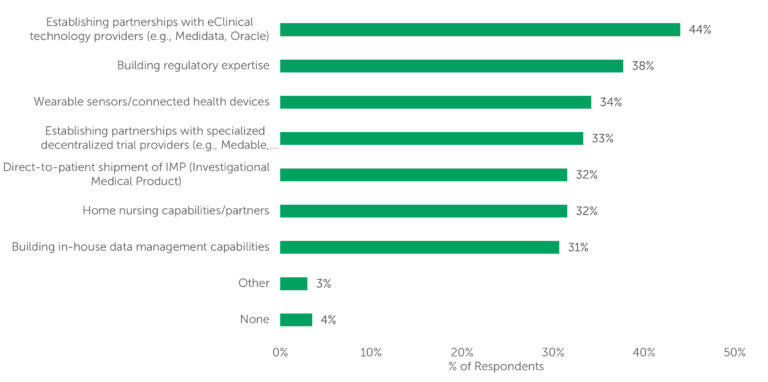
Fig. 1 — “In what areas related to decentralized trials is your company investing significant resources? Please select all that apply” (n=114)
Some components utilized in clinical trials (e.g., wearable sensors) lend themselves well to decentralization. About 42% of respondents report currently utilizing “remote monitoring,” while 55% of respondents expect their “remote monitoring” use to increase in the next two years. Approximately 20% of respondents indicate use of “remote screening,” “direct-to-patient shipment of IMPs,” and/or “remote eConsent,” enabling the trial to progress no matter where the participants are located. In fact, respondents said they expect increased use of all DCT tools and strategies discussed over the next two years (Fig. 2).
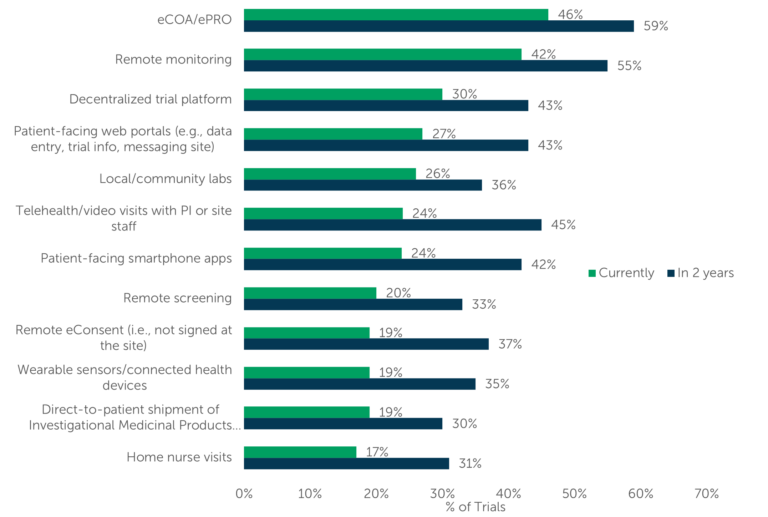
Fig. 2 — “What percentage of your company’s current clinical trials utilize the following components?
What percentage of your company’s clinical trials will utilize these components in two years?” (n=114)
Contributing to this trend is the proliferation of wearable sensors. For example, research suggests more than 200 million Americans use smart watches,[iii] devices capable of collecting real-time data on biological processes, such as heart rate or oxygen levels. Collecting and incorporating patient data using such technologies enriches clinical trials and eases patient burden.
Cost Predictions
When ISR asked respondents for the first time, in the second quarter of 2021, their prediction for DCT costs in two years, nearly half of respondents predicted the cost would be less than traditional trials. But, in ISR’s most recent survey on DCTs (conducted during Q3, 2022), the majority of respondents (65%) stated they believe DCTs are more expensive than traditional trials, while another 20% of respondents stated they consider DCTs and traditional trials to have similar costs (Fig. 3).
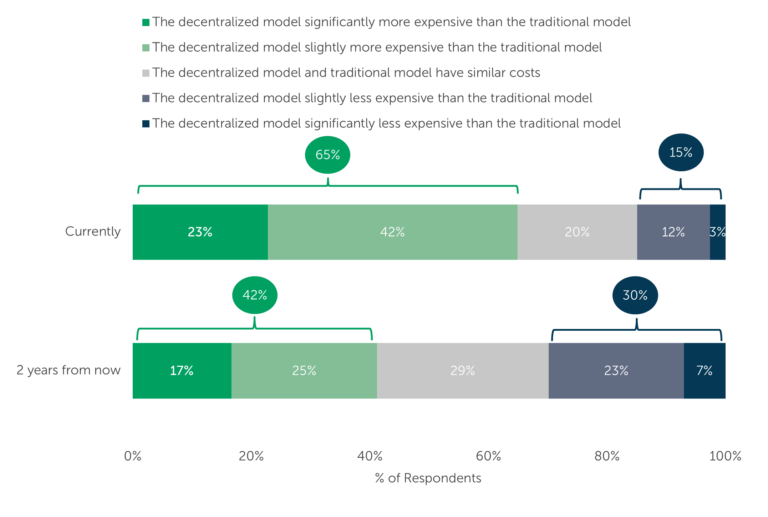
Fig. 3 — Top: “Currently, how do the costs of the decentralized trial model and the traditional model compare?” (n=114)
Bottom: “Two years from now, how will the costs of the decentralized trial model and the traditional model compare?” (n=114)
Respondents to the more recent survey also were split on their belief whether the cost of DCTs will increase or decrease over the next two years, though optimism reigns. The 65% of respondents who currently consider decentralized trials to be more expensive than the traditional model drops to just 42% who believe DCTs will remain more expensive in two years.
The sudden, abrupt shift to partial or fully remote work deeply impacted the clinical trials industry, but we have emerged from the pandemic with useful tools and strategies relevant to DCTs, as well as valuable lessons learned. We have evidence that incorporating remote aspects into clinical trials effectively can contribute to cost savings, enhanced efficiency, higher quality data, a deeper and more diverse patient pool, and the ability to meet more ambitious timelines.
Surveyed pharmaceutical professionals have predicted DCT costs will continue to decrease, leading respondents to invest resources in DCTs more enthusiastically. Thus, not only are inherently decentralized components here to stay, their use should be expected to expand as benefits continue to be identified and are leveraged to greater effect.
[i] World Health Organization, https://www.who.int/europe/emergencies/situations/covid-19
[ii] ISR Reports, Dec. 2022, https://research.isrreports.com/reportaction/2022-decentralized-clinical-trials-market-outlook/Marketing
[iii] Ruby, Daniel. “Smartwatch Statistics 2023,” https://www.demandsage.com/smartwatch-statistics/


