Technology advances so rapidly, it seems that adapting to innovative technology takes more time than it does to actually develop the technology. It was fewer than thirty years ago that we first heard those grating sounds of dial-up internet, connecting just long enough to send an e-mail (electronic mail! How cool, we said). Fast-forward to today, and the streets have self-driving cars, we carry little computers in our pockets that can search the internet in seconds, and there are even robot hostesses that will lead you to your table when you dine out with family or friends. There is no question of the rapid advancement of technology.
When it comes to clinical trials for drug development, Industry Standard Research (ISR) wanted to understand the utilization of some of these technologies – such as patient-facing smartphone apps and wearables. We also wondered about the uptake in using and/or adapting to newer technology in the face of a global pandemic. Our Decentralized Clinical Trials Market Outlook study provides a larger overview, beyond the scope of technology. Understanding the utilization of, challenges with, and improvements needed for the technology in decentralized trials is just one aspect.
Promising Benefits of Decentralized Clinical Trials
Decentralized clinical trials, also sometimes known as virtual or remote trials, have emerged as a promising approach to improve the efficiency, patient experience, demographic diversity, and cost-effectiveness of clinical research. These trials leverage innovative technologies to allow patients to participate in clinical trial activities from the comfort of their homes. During the third quarter of 2022, ISR collected data from biopharmaceutical professionals who were involved in at least one decentralized clinical trial within the past 12 months. Respondents in this study estimate, on average, that their companies are currently conducting 38% of trials using the decentralized model. They expect this to rise to 50% in the next two years. Among the various technologies used in decentralized trials, electronic Clinical Outcome Assessments (eCOA) and electronic Patient Reported Outcomes (ePRO), as well as wearable devices, are examples of technologies that have been gaining attention as potential game-changers in the field.
eCOA/ePRO technologies refer to electronic systems that allow patients to report their outcomes, symptoms, and other relevant data remotely, using smartphones, tablets, or computers. These technologies offer several advantages over traditional paper-based methods, including increased accuracy, real-time data capture, and reduced burden on patients and site staff. In decentralized trials, eCOA/ePRO technologies can help to ensure that patients stay engaged and comply with study requirements, even if they are not physically present at a study site. Respondents in our research reported use of eCOA/ePRO most often in recent decentralized trials (46% of their company’s trials, on average), and they expect increased use over the next two years (see Figure 1).
Wearable devices, such as smartwatches or activity trackers, have also become increasingly popular in decentralized trials. These devices can collect various types of physiological data, such as heart rate, sleep patterns, or activity levels, and transmit them to study databases in real-time. This can help to provide more objective, continuous, and comprehensive data than traditional methods, as well as improve patient adherence and motivation. Respondents indicated 19% of their company’s recent decentralized trials involved wearables but expect this to increase to 35% of their trials in the next two years (see Figure 1).
Wearables are among those remote components currently utilized least often, and yet, even among the least-utilized, these components are reported to be implemented in nearly one out of five decentralized trials, according to our respondents. Overall, use of each of these components is expected to increase over the next two years.
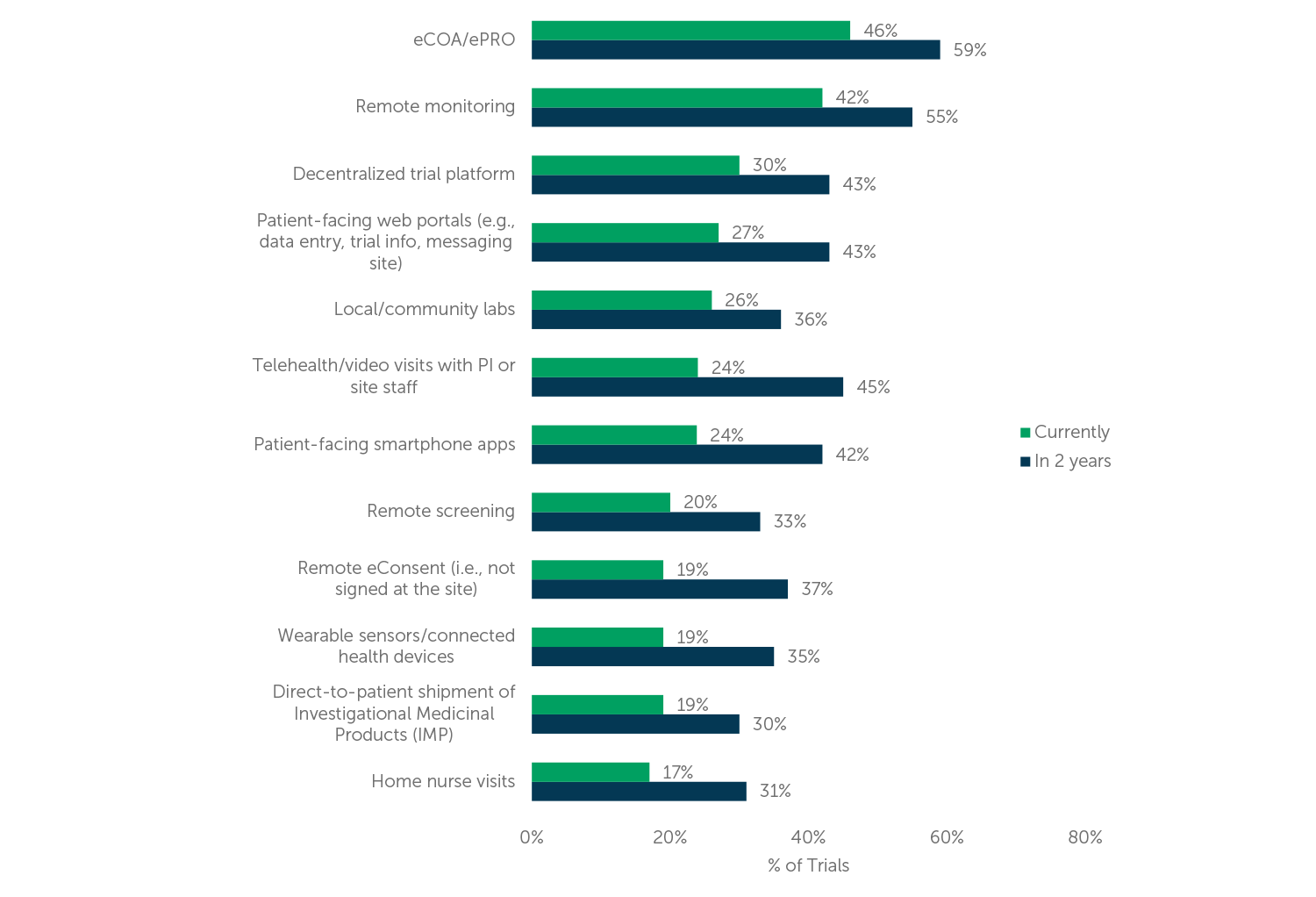
Figure 1 (n=114) “What percentage of your company’s current clinical trials utilize the following components? What percentage of your company’s clinical trials will utilize these components in two years? Your best estimates are fine.”
Challenges with DCT Technologies
While decentralized trials offer many benefits, there are still several areas in which technology needs improvement to fully realize its potential. 66% of respondents believe that currently available clinical technologies only “somewhat”, “slightly” or “not at all” meet their decentralized trial needs, leaving room for improvement in this area. The top two technologies needing improvement, according to respondents to our 2022 survey, are Patient-facing web portals and Wearable sensors (see Figure 2).
While our full report offers more detail from respondents, the key takeaways about technology improvement revolve around broader themes of ease of use and reliability. Decentralized trials generate large amounts of data that must be accurately collected, managed, and analyzed. One challenge is ensuring the validity and reliability of electronic data. Validation of the electronic devices and software used in decentralized technological components is critical to ensure that the data collected are accurate and reliable.
Another challenge is patient compliance with electronic data collection. While many of the decentralized components/technologies can help to reduce patient burden, some patients may struggle with the use of electronic devices or may have concerns about the privacy and security of their data. It is important to ensure that patients receive adequate training and support to use the electronic devices and software effectively and that patient privacy and data security protocols are in place.
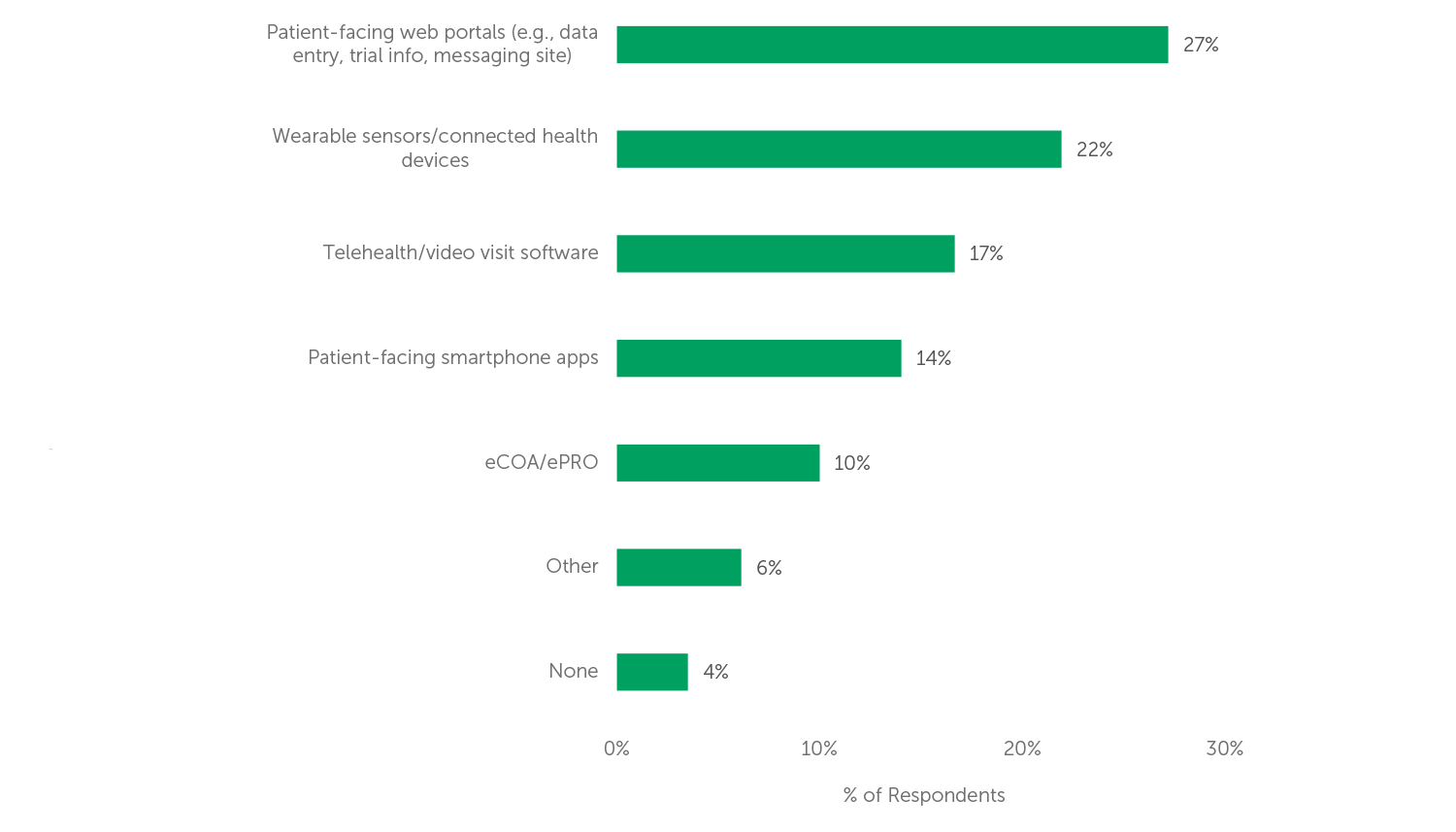
Figure 2 (n=114) “Which technology needs the most improvement to meet your decentralized trial needs?”
Altogether, decentralized clinical trials offer many benefits, but there are still several areas in which technology needs improvement. Data collection and management, reliability and ease of use, and data security are just a few areas in which technology can play a vital role in improving the decentralized trial process. By addressing these challenges, technology can help facilitate the adoption of decentralized clinical trials and help to advance clinical research. Market research data in this article was powered by the ISR Health Panel. Want to contribute to thought leadership pieces and help to make the pharma industry better? Join the Health Panel today.





2 Comments