Sponsors can facilitate successful CRO partnerships by clearly defining the goals of the clinical trial, choosing an outsourcing model that considers project needs and internal capabilities/resources appropriately, communicating consistently and effectively during the decision-making process, and informing provider selection with trustworthy market research. With these steps in mind, drug developers can foster fruitful outsourcing relationships with clinical trial providers that will best support their business goals.
1. Assess the Goals and Nuances of the Clinical Trial
Clearly describing a clinical trial’s intricacies and potential challenges will allow sponsor companies to proceed with a more informed approach to CRO selection. This helps ensure that sponsors are well-equipped to communicate their trial’s nuances to potential CROs and evaluate a provider’s ability to effectively meet their needs.
Define Trial Objectives – Determine the specific goals, endpoints, and expected outcomes of the clinical trial. What does a successful trial look like for your project?
Assess Trial Complexity – Evaluate the trial’s complexity in terms of patient population, therapeutic area, trial phase, geography, and regulatory requirements.
Identify Key Challenges – Explore potential challenges such as patient recruitment difficulties, data management needs, regulatory hurdles, and any unique aspects of the trial.
Develop a Request for Proposal (RFP) – Draft a project overview that clearly communicates your expectations to CROs, outlining the trial’s objectives and scope of work. Make sure to include the nuances of patient recruitment, regulatory requirements, data management, budget, and timelines.
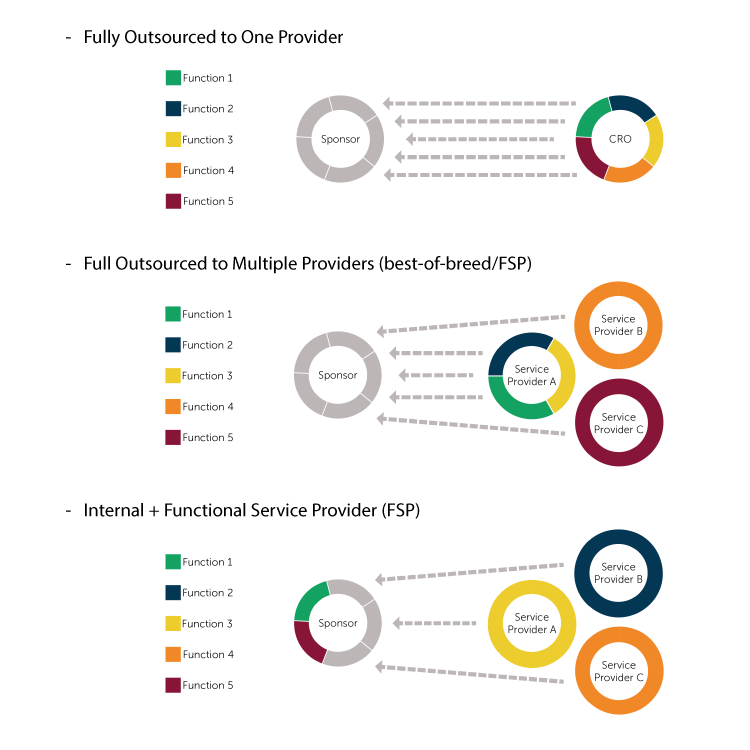
2. Choose the Right Outsourcing Model for the Project
Top level drivers like internal resource levels, patient recruitment strategy, and therapeutic expertise can direct a drug developer towards the outsourcing model best suited for a specific trial. Outsourcing strategies in the biopharmaceutical sphere include:
3. Communicate Throughout the Decision-Making Process
Bring all players to the table to discuss selection criteria, project goals and timelines, available resources, and input from key decision-makers. Here are some of the key roles and responsibilities involved in trial design and execution:
Decision-making Roles
- Clinical Operations (including clinical monitoring, clinical project management, etc.)
- Data Management
- Executive Management
- Functional Leads (e.g., biostatistics, technology, safety, regulatory)
- Medical Director
- Outsourcing/Procurement Representative
- Research & Development
- Therapeutic Area Head
Decision-making Responsibilities
- Allocating budget
- Choosing CROs to invite to bid
- Deciding to outsource
- Directly managing CROs
- Evaluating and selecting CROs
- Managing clinical operations
- Setting study objectives (designing trial protocol, etc.)
4. Identify Your CRO Selection Criteria
Understanding and discussing the needs of the company, the project, and strengths of the CRO can foster a successful and efficient drug development process. Acknowledgement of resources internally and of the CRO enables contract researchers to build complimentary teams with the ability to meet requirements. Here are a few of the most important CRO selection attributes according to ISR’s respondents from sponsor organizations:
- Expectations for data quality
- Experience with similar study types
- Global footprint
- Low cost
- Metrics for meeting overall project timelines
- Offers innovative solutions
- Operational excellence
- Patient recruitment strategy
- Prior positive experience with service provider
- Therapeutic expertise

5. Utilize Market Research, Experiential Data, and Peers’ Ratings to Choose the Best CRO for Your Project
The CRO landscape is not small and selecting the best CRO for your project is no easy task. Utilizing performance benchmarking studies, drawing upon the experience of your peers, and hearing verbatim reasons for why recent users of CROs rated their satisfaction with a CRO as they did, will lead you to more informed decisions. With Industry Standard Research studies, uncover and learn:
- Satisfaction and performance ratings for more than 30 CROs for Phase I and Phase II/III studies
- Familiarity, reported usage rates, and perceptions of leadership for multiple CROs
- Your peers’ preference of CRO if the choice were entirely up to them
By strategically managing outsourcing relationships with service providers, drug sponsors can encourage the success of their clinical trials and potentially form lasting partnerships. Explore the reports below to learn more about the importance of developing an outsourcing plan, anticipated future trends in outsourced clinical development, and benchmarking CRO performance to be as informed as possible when selecting a service provider: