Insights from Recent eCOA/ePRO Users
This year ISR Market Research released the sixth edition of our eCOA/ePRO Benchmarking & Market Dynamics report. Electronic clinical outcome assessment and patient reported outcomes systems allow clinicians, patients, and caregivers to report clinical trial outcomes electronically, streamlining data collection and improving the patient experience. ISR asks users of these eCOA/ePRO systems (pharma sponsors and CROs) to share their opinions on a variety of topics, including the criteria that are most important in choosing an eCOA/ePRO provider, perceptions of the various solutions on the market, and more.
With each subsequent round of survey research on clinical technology solutions, ISR captures data on improvements users would like to see to their technology systems and aims to identify trends that will be useful as the industry works to develop the next generation of solutions. Though certainly not a new concept, the findings in this edition of the report illustrate the deepening importance of patient-centric technology design in the eCOA/ePRO space.
Top eCOA/ePRO Selection Attributes
Respondents were asked to share the five criteria most important to them when choosing a provider for eCOA/ePRO services. Ease of use for patients featured among respondents’ top five selection criteria most frequently, with nearly half of respondents (46%) including this attribute among their top 5. (See Figure 1).
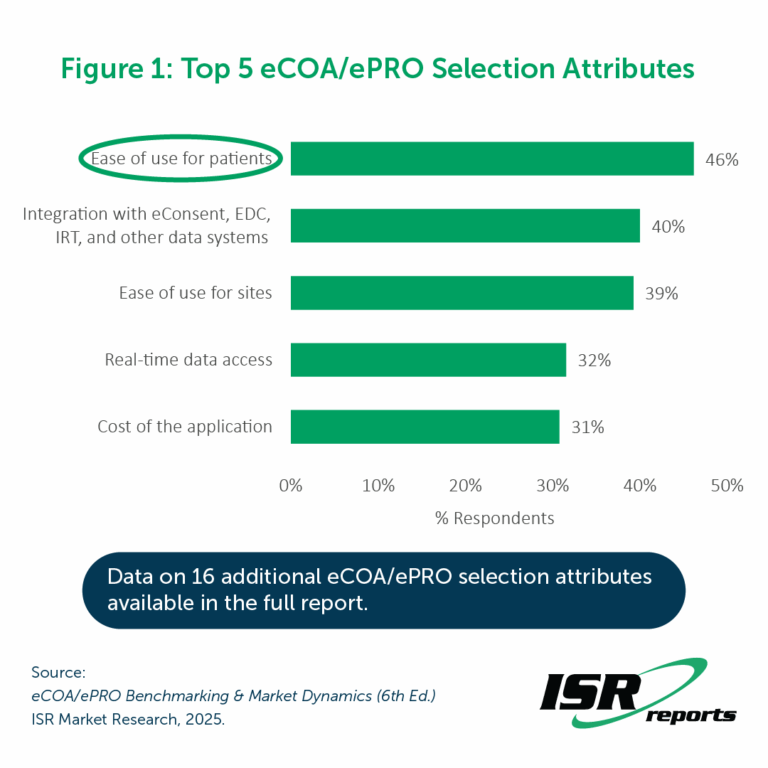
Similarly, when looking at the attributes respondents expect to become more important as they evaluate eCOA/ePRO systems over the next 12 months, Ease of use for patients was second among attributes expected to become more important, ahead of 19 other attributes. The importance of the patient experience cannot be overstated – cumbersome technology systems could prevent patients from completing critical assessments, contribute to lower patient retention in clinical trials, and ultimately affect clinical trial data quality.
We imagine this feedback is not surprising to technology providers. While noteworthy – Ease of use for patients stands out in this report compared to our other research on other clinical trial technology systems (e.g., EDC, CTMS, and IRT) – eCOA/ePRO providers have been focusing on the patient experience for years now. How can they differentiate themselves?
Patient Owned Devices
We hear consistently about the need to make systems compatible with patient-owned devices (BYOD) – the idea is that patients will be more familiar with their own smartphones, tablets, etc. than a device provided for the clinical trial which could ensure higher compliance with answering questionnaires and better patient safety outcomes. The BYOD approach is also more cost effective than using vendor-supplied devices and avoids challenges with distributing and supporting those devices. Therefore, we ask respondents to report the digital sources of reported outcomes over the past two years and what they would expect to see over the next two years. On average, respondents predict an increase in usage of Patient-owned devices (smartphones, tablets, etc.) to report outcomes (from 20% over the past two years to 31% over the next two years) alongside a slight decrease in other approaches.
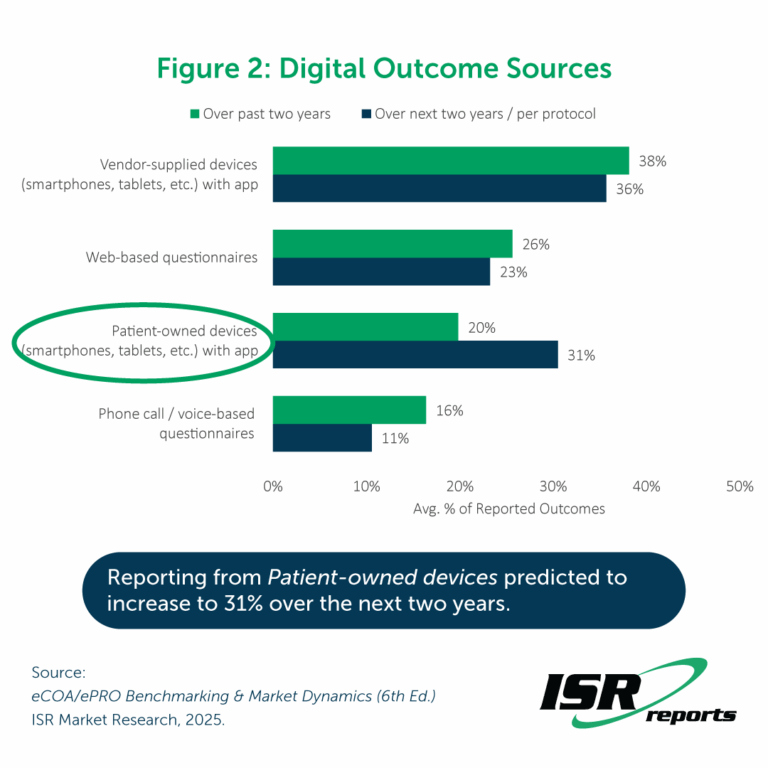
Interestingly, despite optimism that the proportion of outcomes reported on patient-owned devices will increase, these numbers have not budged from (and even slightly underperformed) the predictions from two years ago. In 2023, respondents reported that an average 21% of the outcomes reported over the prior two years had been from patient-owned devices and predicted that number to increase to 35% over the next two years. Why this sluggishness? It is likely very challenging to develop and maintain a system that will function the same way on many different devices and provide support for sites and patients encountering issues with data collection. There may also be some hesitance around regulatory acceptance of BYOD. But for eCOA/ePRO providers, investing more in systems compatible with patient devices could end this stagnation and help to differentiate their solutions from those of their competitors.
Patient Reported Outcomes
Lastly, we ask respondents to share the proportion of COAs (clinical outcome assessments) coming from patients, clinicians, and caregivers over the past two years and to predict how this proportion will change over the next two years. Survey respondents predict an increase in the proportion of COAs coming from patients, from an average of 41% over the past two years to 48% over the next two years. On the other hand, respondents predict a decrease in COAs coming from clinicians and for the proportion of COAs coming from caregivers to remain roughly the same. This shift towards patient reported outcomes means there will be greater pressure on ePRO systems to be intuitive for patients to use and compatible with patient devices.
eCOA/ePRO Benchmarking and Market Dynamics (6th Ed.)
The team at ISR is looking forward to seeing how patient-facing clinical trial technologies continue to develop over the next few years. Please let us know if you have any questions about this edition of our eCOA/ePRO report or ideas for further research by reaching out to info@isrreports.com. You can also register an account on our website and follow us on LinkedIn for more information about this report and other primary market research available in our library.
Primary market research data in this article were powered by the ISR Life Science Panel. Want to contribute to thought leadership pieces and help to make the pharma industry better? Join today.





