Optimizing Feasibility in Clinical Trials
$2,460.00 – $4,920.00
February 2012
Licensing Options
- SINGLE-USER LICENSE
A Single-User License allows access to an individual user.
- ENTERPRISE-WIDE LICENSE
An Enterprise-Wide License allows access to all employees and sites within an organization.
Report Overview
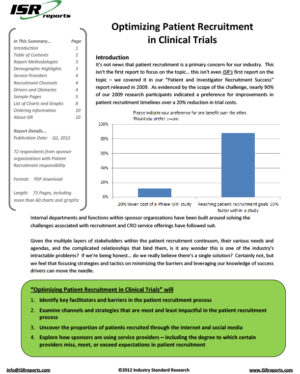
Patient recruitment has been and will likely continue to be one of the most challenging steps in the clinical development process. Sponsors need accurate feasibility estimates to know how long it will take to bring a product to market, how much it will cost to get to approval, and if it is possible to recruit the necessary volume of patients in order to collect data with sufficient statistical power to meet regulatory requirements. For these feasibility estimates, they rely heavily on CROs and investigators who will, through the use of neglected databases and draft protocols with incomplete inclusion/exclusion criteria, promise the moon in an attempt to win business.
What you will learn:
- Whether the state of feasibility in clinical trials different than it was when we addressed this topic in 2009?
- Are there any organizations doing it meaningfully better than others? If so, who? And what are they doing?
- How, if at all, has the industry embraced available technologies
- How would sites and pharma personnel like to see feasibility change?
Report Contents:
- Clinical Study Feasibility – Current State: Discusses the current state of feasibility from the perspective of clinical trial sites and study sponsors – Who does it best? Is any organization truly differentiated? What improvements are necessary?
- Feasibility Going Forward – Here ISR examines the past, current and future use of various technologies including electronic health records, prescription databases, and more.
-
Relationships – Sites, CROs, Sponsors – Feasibility is not a solitary activity. It depends on the systems, capabilities, planning, and honesty of all three stakeholders. This section examines how CROs and sites are used in the process and specifically rates the accuracy of various CROs’ feasibility studies.