Interactive response technology (IRT) systems are used to automate supply management, randomization, and analytics for clinical trials. They ensure participants receive the correct treatment at the correct time, allow for enhanced drug supply logistics tracking, and empower users to obtain real-time data from participants throughout the study. Accordingly, selecting the right IRT system is critical to trial success.
To create Industry Standard Research’s October 2022 report, IRT Market Dynamics and Service Provider Benchmarking (4th edition), we asked the decision makers at sponsor organizations and CROs to discuss the criteria they consider when selecting an IRT system. While the full report covers specific provider metrics in more detail (which IRT solutions are most frequently used, how they perform in key service areas), it also can be fruitful to learn how others in the outsourcing community are evaluating IRT providers.
Integration with EDC, ePRO, CTMS and other data systems topped respondents’ selection criteria by a large margin; it was selected by nearly two-thirds of respondents (61%) as one of their “Top 5” criteria (Fig. 1). This is not surprising since, within the clinical technology ecosystem, the importance of integration across platforms cannot be overstated as data quality is paramount to clinical trial success.
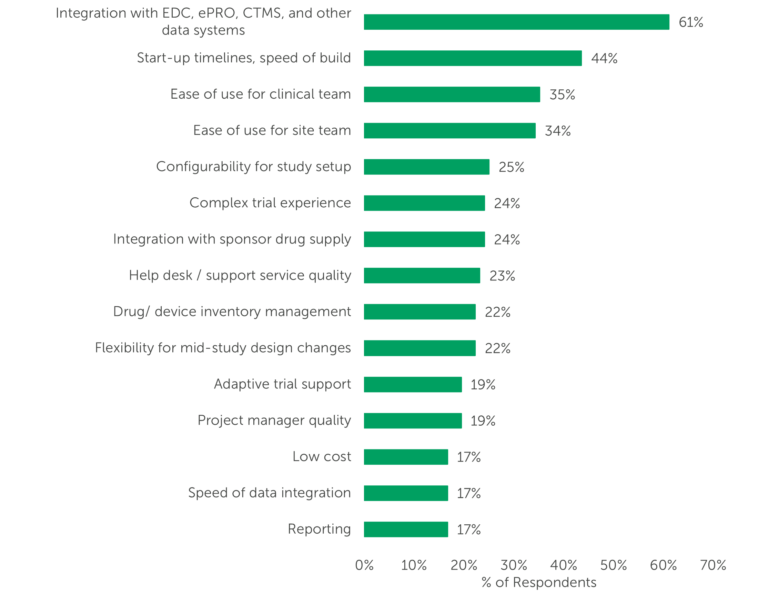
Figure 1 – “Please review the following attributes and select the five most important to you when selecting a provider for IRT services.” NOTE: (n=108) Only responses included in the “Top 5” by ≥15% of respondents are shown.
Ease of use, for both the clinical team and the site team, was also highly rated, included among the “Top 5” attributes by more than one-third of respondents. Several aspects of the timeline and system build customization — including Start-up timelines, speed of build (44%), Configurability for study setup (25%), and Flexibility for mid-study design changes (22%) — also emerged as important selection criteria.
Another highly valued attribute in the IRT selection process is the ability to support a Complex trial experience. While only about 24% of survey respondents listed it among their “Top 5” most important attributes, nearly 9-in-10 respondents reported selecting different vendors for less complex vs. more complex builds at least some of the time, and very few indicated that they always use the same IRT vendor regardless of the study build complexity (13%). These findings indicate that sponsors and CROs are seeking IRT service providers that can configure the IRT system to accommodate unique trial needs and support differing levels of complexity.
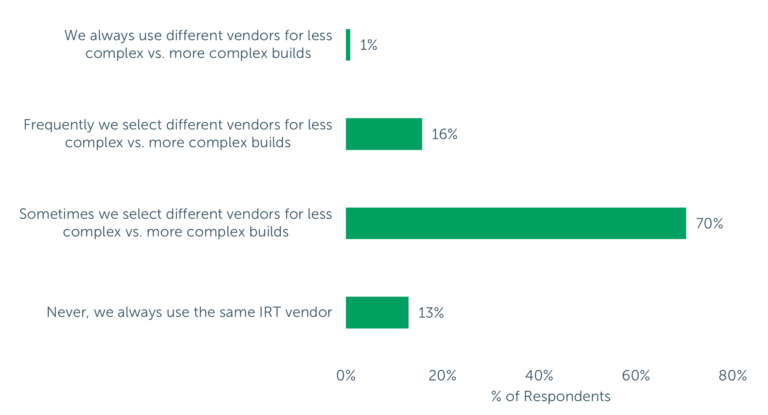
Figure 2 – “How often do you select different IRT vendors based on the complexity of the study build?” NOTE: (n=108) On average, respondents consider 39% of trials to be moderately complex and 36% of trials to be highly complex.
Expanding on the role that trial complexity plays in vendor selection, respondents reported that they consider an average of three-quarters of trials to be moderately or highly complex. The reality is that even seemingly straightforward or early-phase trials might have complex randomization and trial supply management (RTSM) needs.
Therefore, providers that can accommodate highly complex studies set themselves apart from competitors. However, additional complexity often is accompanied by additional cost. While outsourcers generally value a number of selection criteria more highly than low cost (per Fig. 1, 17% of respondents selected Low cost as a “Top 5” concern), larger IRT providers may lose out to providers that offer a “no frills” model on the less complex studies.
Trends Moving Forward
We expect IRT providers (including CROs that offer IRT services, dedicated IRT providers, and large integrated technology service providers) to continue focusing on integration with other clinical technologies — one of the industry’s greatest challenges. Although integration certainly has improved, the outsourcing community continues to experience pain points managing multiple systems and thus prioritizes integration over many other selection criteria. This trend is exacerbated by clinical trials’ ever-increasing complexity.