Optimizing Investigator Recruitment in Clinical Trials
Price range: $2,460.00 through $4,920.00
May 2012
Licensing Options
- SINGLE-USER LICENSE
A Single-User License allows access to an individual user.
- ENTERPRISE-WIDE LICENSE
An Enterprise-Wide License allows access to all employees and sites within an organization.
Report Overview
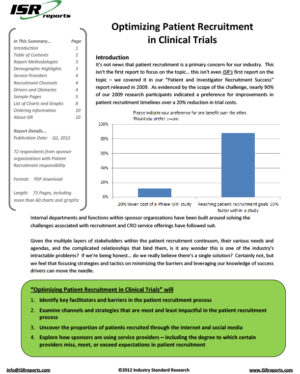
This report is based on a survey of 116 pharmaceutical decision-makers specifically responsible for investigator recruitment activities. The report uncovers drivers and barriers in the investigator recruitment process, identifies a gap between what is currently being done and what needs to be done to grow the pool of qualified PIs, outlines leading-edge strategies the industry is using to address these challenges, and discusses the use of CROs – including where they are missing, meeting, and exceeding sponsors’ expectations – to help pharmaceutical professionals optimize their investigator recruitment activities.
What you will learn:
- Key drivers and obstacles in the investigator recruitment process. This allows project teams to prepare for – and mitigate – delays.
- The gap between what’s happening and what’s required to grow the pool of qualified investigators. Convince internal audiences to invest in growth strategies.
- CRO recruitment effectiveness. Which service providers lead and which lag in the battle to recruit investigators?
- Investigator database quality. Which CROs possess the most productive investigator databases (Overall, Cardiology, CNS, and Oncology)?
Report Contents:
- Drivers and Obstacles to Investigator Recruitment Success
- Recruitment Solutions, including: best methods to find new investigators, geographical challenges, and site identification strategies
- Use of External Service Providers, including: CRO use, CRO site selection effectiveness, and investigator database productivity by therapeutic area