[account_shop_links]
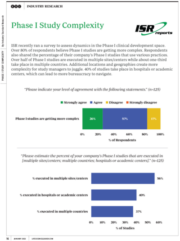
ISR recently ran a survey to assess dynamics in the Phase I clinical development space. Over 80% of respondents believe Phase I studies are getting more complex. Respondents also shared the percentage of their company’s Phase I studies that use various practices. Over half of Phase I studies are executed in multiple sites/centers while about one-third take place in multiple countries. Additional locations and geographies create more complexity for study managers to juggle. 40% of studies take place in hospitals or academic centers, which can lead to more bureaucracy to navigate.
View Resource
